Antennova Presents New CD73 Inhibitor Data with 89.5% DCR at ESMO 2024
BOSTON, MA, USA I September 16, 2024 I Antennova, a biotechnology company at the clinical stage with an emphasis on oncology, revealed that new data concerning the CD73 small molecule inhibitor ATN-037 were shared through a Mini Oral presentation at the 2024 European Society of Medical Oncology Congress (ESMO Congress 2024).
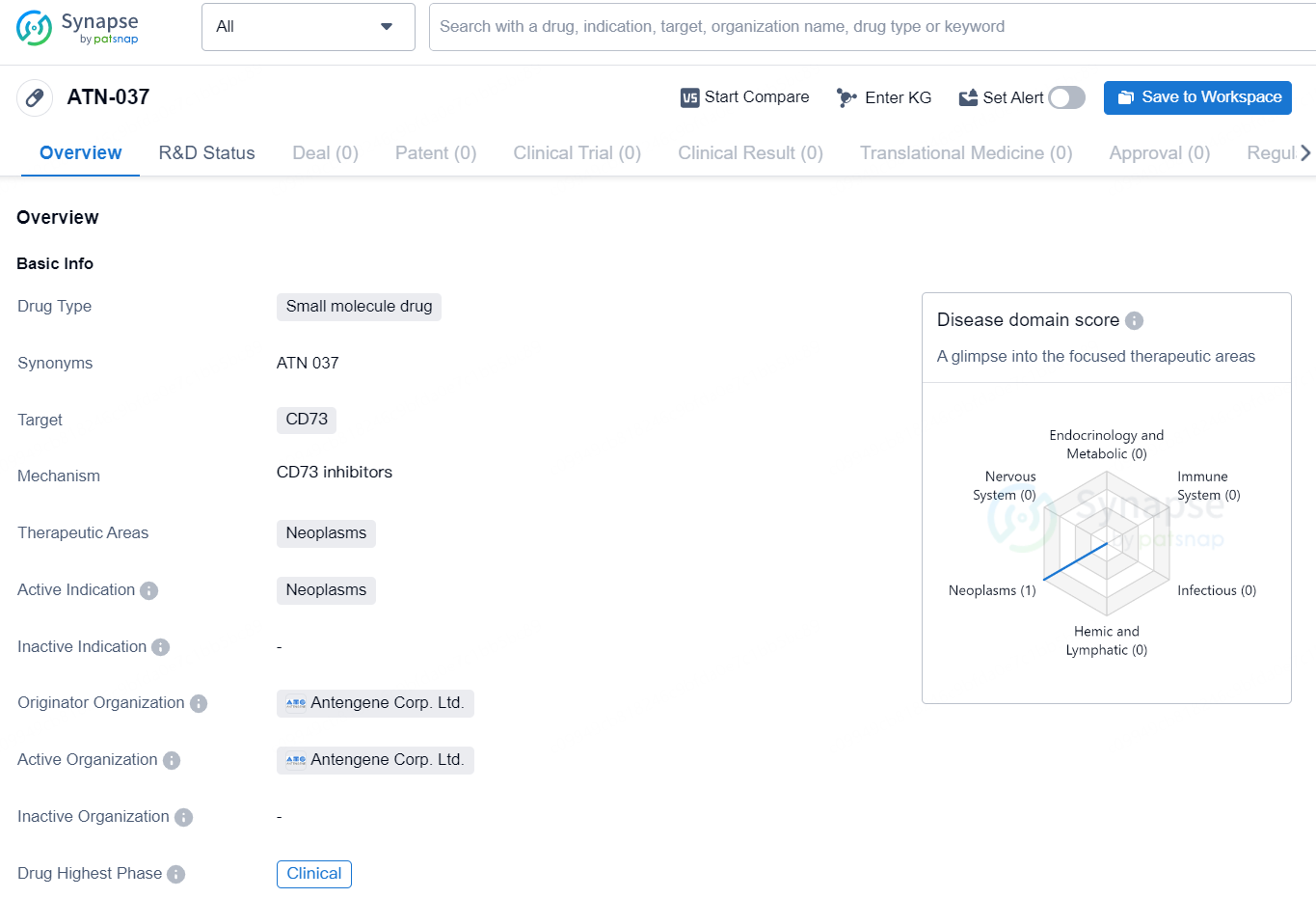
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Antennova, a Delaware-based corporation and a subsidiary of Antengene (HKEX: 6996), is a clinical-stage biotech firm focused on developing groundbreaking treatments that target key biological mechanisms, allowing cancers to evade and resist existing therapies. Antennova is advancing a portfolio of oncology candidates aimed at boosting the efficacy of standard treatments, overcoming checkpoint inhibitor (CPI) resistance, and attacking "cold tumors" unresponsive to current CPI therapies.
ATN-037 is a potent oral small molecule that inhibits CD73. The STAMINA-01 Phase I/II trial was designed to assess the safety, pharmacokinetics, and optimal dosing of ATN-037, either as a standalone treatment or in combination with Merck's (known as MSD outside the U.S. and Canada) anti-PD-1 therapy KEYTRUDA® (pembrolizumab) in patients with refractory or relapsed solid tumors. Antennova has commenced the dose optimization and expansion phase of the Phase II STAMINA trial of ATN-037 in Australia and plans to start the study in China by late October 2024.
As of July 26, 2024, 43 patients have been enrolled and received ATN-037 monotherapy. Among them, 26 patients with acquired CPI resistance were given ATN-037 along with pembrolizumab. Since treatment initiation (C1D1), 42 patients have undergone at least one tumor evaluation (1 patient was unevaluable).
Efficacy data reveal that out of the 42 evaluable monotherapy patients, 23 attained stable disease (SD). Among the 26 evaluable patients treated with the ATN-037 and pembrolizumab combination, 9 had NSCLC and 10 had melanoma. Four of these patients (2 with NSCLC and 2 with melanoma) achieved a confirmed partial response (PR). For the 19 patients with NSCLC or melanoma, the ORR was 21.1% (4/19) and the DCR was 89.5% (17/19).
Throughout the study, all 43 (100%) patients experienced treatment-emergent adverse events (TEAEs), 62.3% of which were treatment-related. Among them, 16 patients experienced treatment-emergent serious adverse events (TE-SAEs), with 2 being treatment-related. Eighteen patients experienced Grade 3 or higher TEAEs, 4 of which were treatment-related. At a dose of 400 mg twice daily (BID), there was one instance of dose-limiting toxicity (DLT) manifesting as a Grade 3 rash. No Grade 5 treatment-related adverse events (TRAEs) were reported.
Preliminary data from the Phase I dose escalation of the STAMINA study have shown promising PR in patients receiving ATN-037 in combination with pembrolizumab, with an excellent safety profile. This combination might offer a new therapeutic option for CPI-resistant NSCLC and melanoma patients.
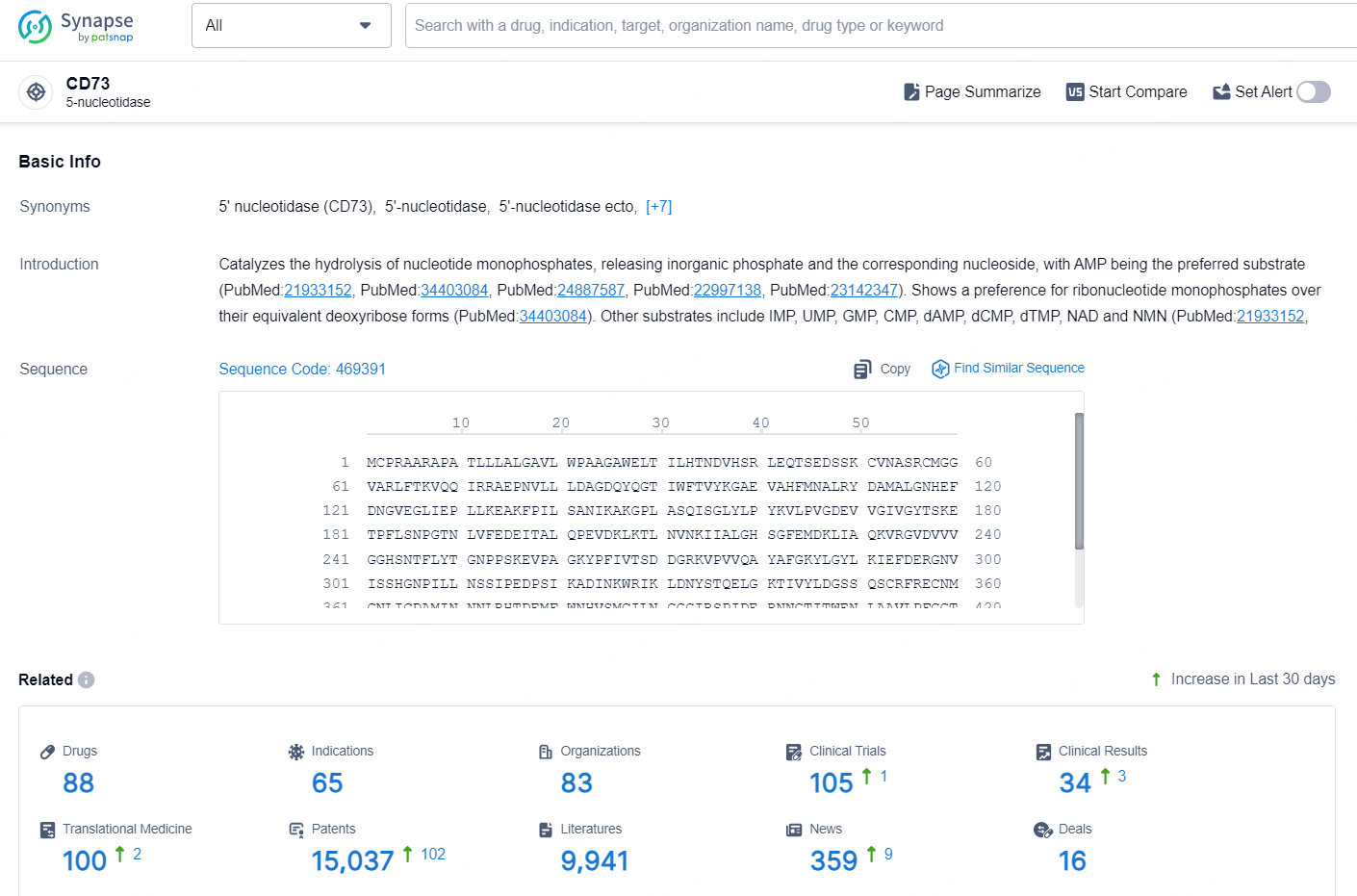
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of September 18, 2024, there are 88 investigational drug for the CD73 targets, including 65 indications, 83 R&D institutions involved, with related clinical trials reaching 105, and as many as 15037 patents.
ATN-037 is a small molecule drug developed by Antengene Corp. Ltd. The drug is designed to target CD73, a protein associated with neoplasms, making it a potential treatment for various types of cancer. Currently, ATN-037 is in the clinical phase of development, indicating that it is being tested in human subjects to evaluate its safety and efficacy.