Anti-Netrin-1 Antibody Shows Potential in Combating Endometrial Cancer
In a new study published in the journal Nature, researchers have unveiled a promising therapeutic strategy for combating endometrial cancer (EC) by blocking the protein Netrin-1. The study, titled "Netrin-1 blockade inhibits tumor growth and EMT features in endometrial cancer," presents compelling evidence of the efficacy of a novel anti-Netrin-1 antibody in reducing tumor progression and inhibiting epithelial-to-mesenchymal transition (EMT).
Blockade of Netrin-1
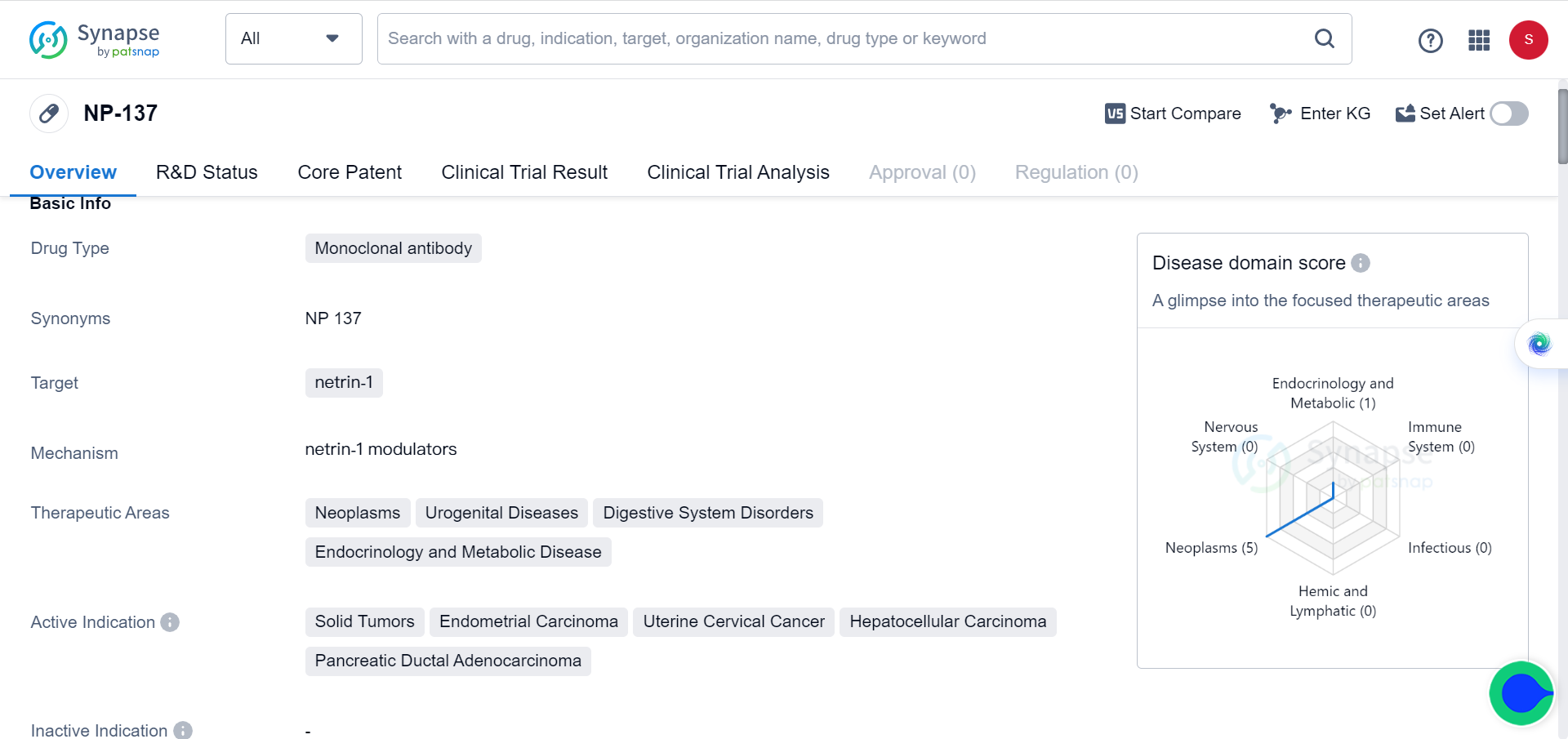
Netrin-1, known for its critical role in neuronal navigation, angiogenesis, and cell survival during embryonic development, has been found to be upregulated in various types of cancers, including endometrial carcinomas. Leveraging this knowledge, the research team sought to investigate the potential of Netrin-1 blockade as a therapeutic approach for EC.
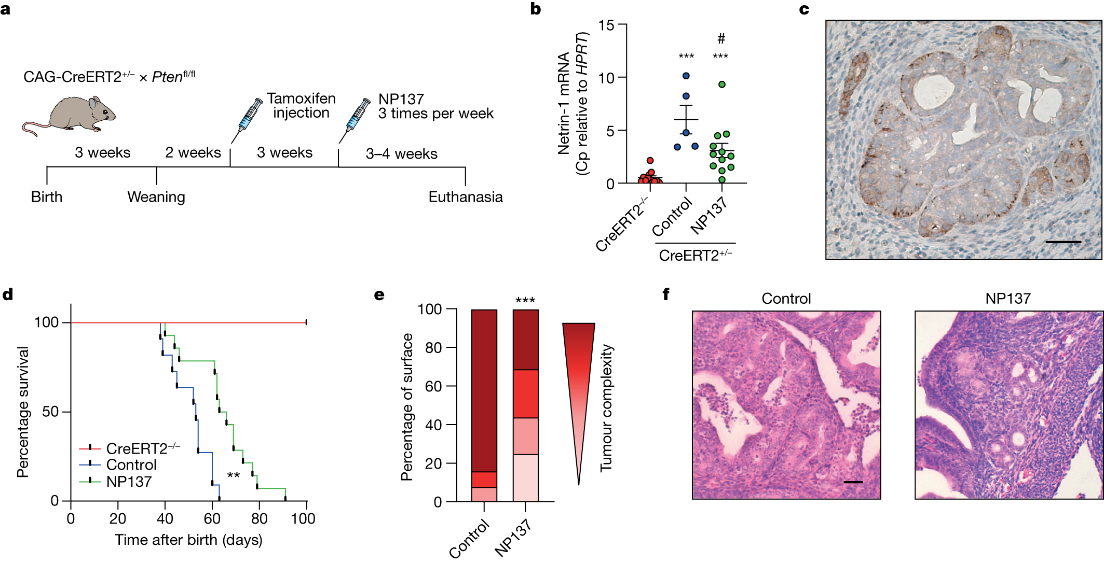
The study commenced with an examination of Netrin-1 upregulation in human endometrial carcinomas, confirming its presence in the majority of cases. To test the effectiveness of Netrin-1 blockade, an anti-Netrin-1 antibody called NP137 was employed in an EC mouse model. The results demonstrated that NP137 effectively reduced tumor progression.
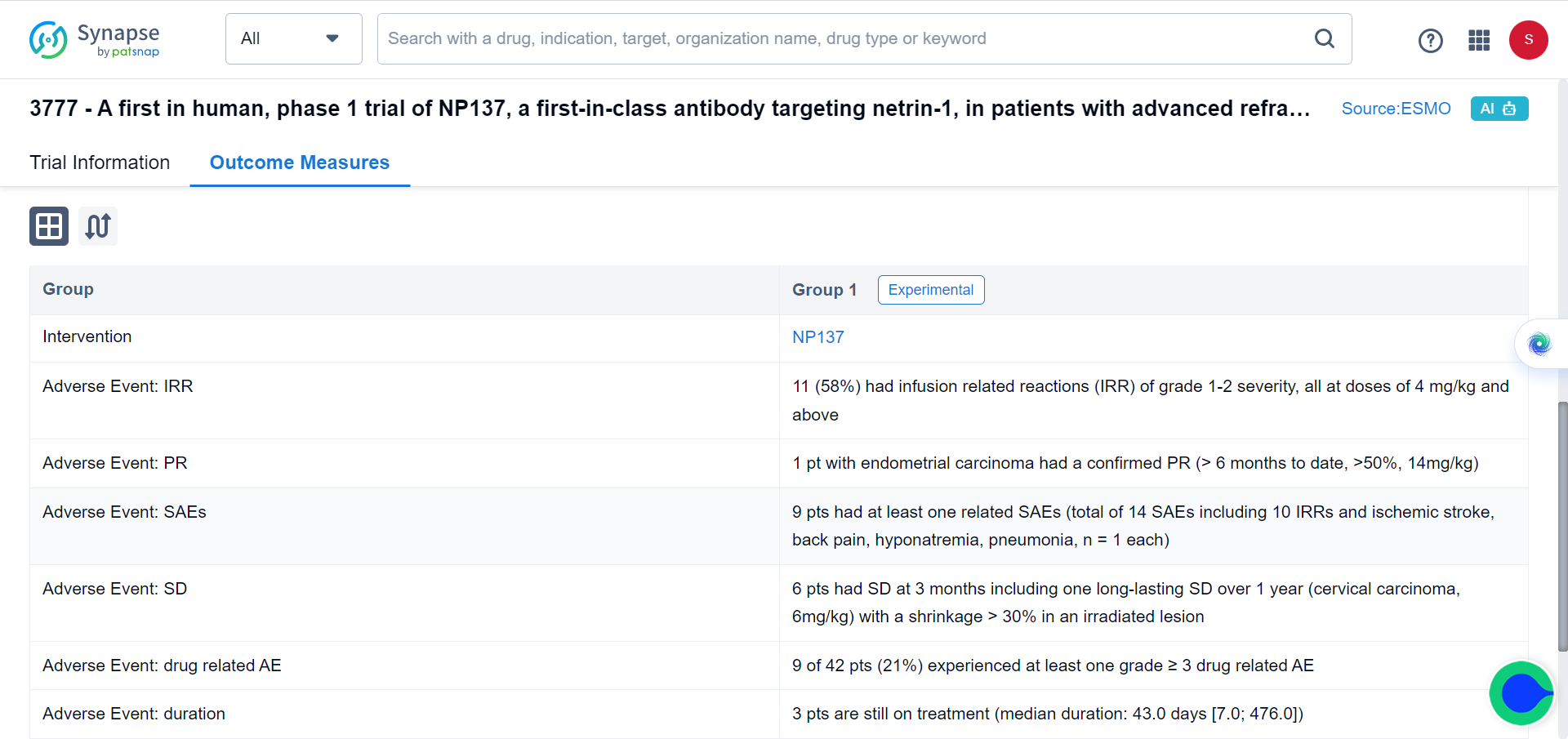
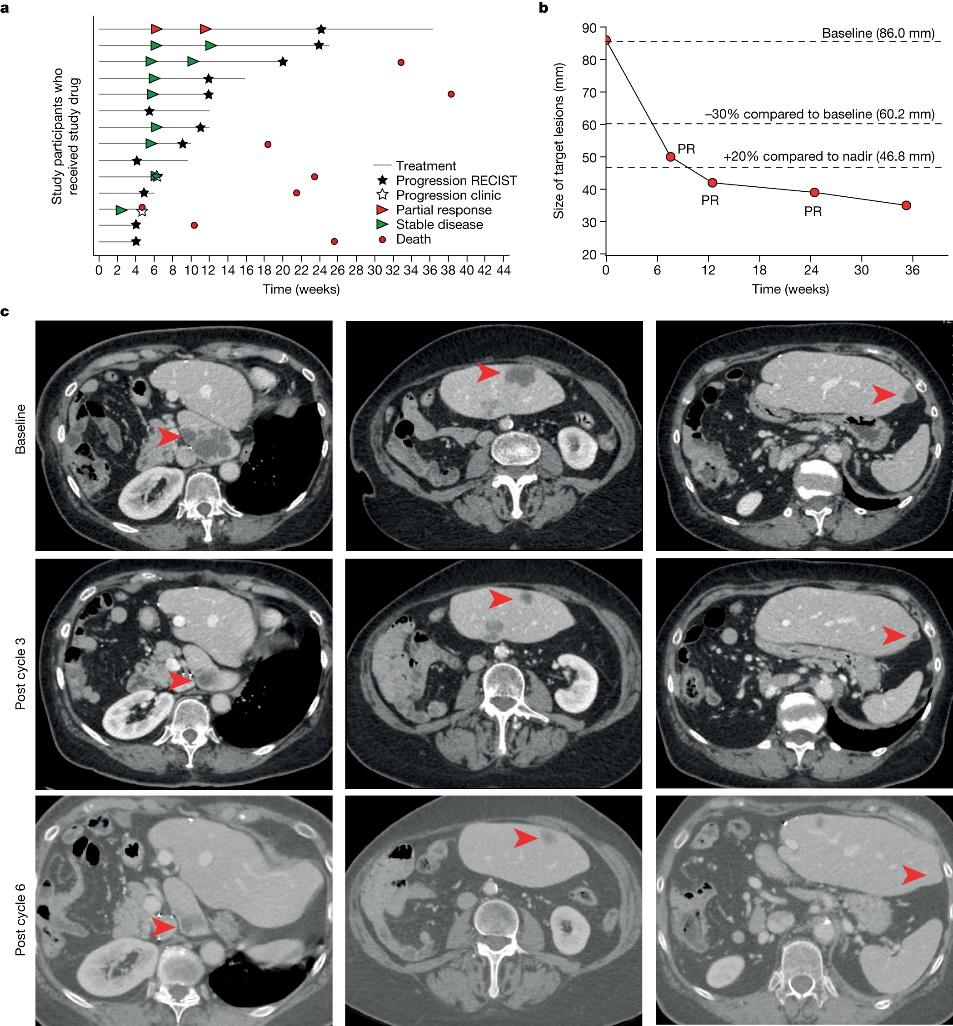
Building on these promising preclinical findings, the researchers conducted a Phase I trial involving 14 patients with advanced EC. Administering NP137 as a single agent, the trial yielded encouraging outcomes, with eight patients experiencing stable disease and one patient exhibiting an objective response as RECIST v.1.1 (Response Evaluation Criteria in Solid Tumours version 1.1, partial response, 1 out of 14, 51.16% reduction in target lesions at 6 weeks) as the best response to NP137 treatment, demonstrating a reduction in target lesions of up to 54.65% over six months.
To gain insights into the mechanism of action of NP137, the researchers performed gene profiling on mouse tumors. The analysis revealed that, in addition to inducing cell death, NP137 inhibited the process of epithelial-to-mesenchymal transition (EMT). To validate these findings in human patients, paired pre- and on-treatment biopsies from EC patients in the NP137 trial were subjected to various RNA sequencing techniques.
The researchers performed bulk RNA sequencing (RNA-seq) on matched pre-treatment and on-treatment biopsies from patients with endometrial cancer (EC) in the NP137 trial to evaluate the mechanism of action of NP137. Bulk RNA-seq is a technique that involves sequencing the total RNA pool from a tissue sample, as opposed to isolating individual cells. The researchers used this technique to identify changes in gene expression patterns in tumor cells before and after NP137 treatment. They found that NP137 treatment led to an overall decrease in tumor epithelial-to-mesenchymal transition (EMT), which was associated with changes in immune cell infiltration and increased interactions between cancer cells and the tumor microenvironment. This suggests that netrin-1 blockade using NP137 could be a promising clinical strategy for inhibiting tumor progression and EMT features in endometrial cancer by reducing netrin-1 signaling.
Combine with carboplatin-paclitaxel
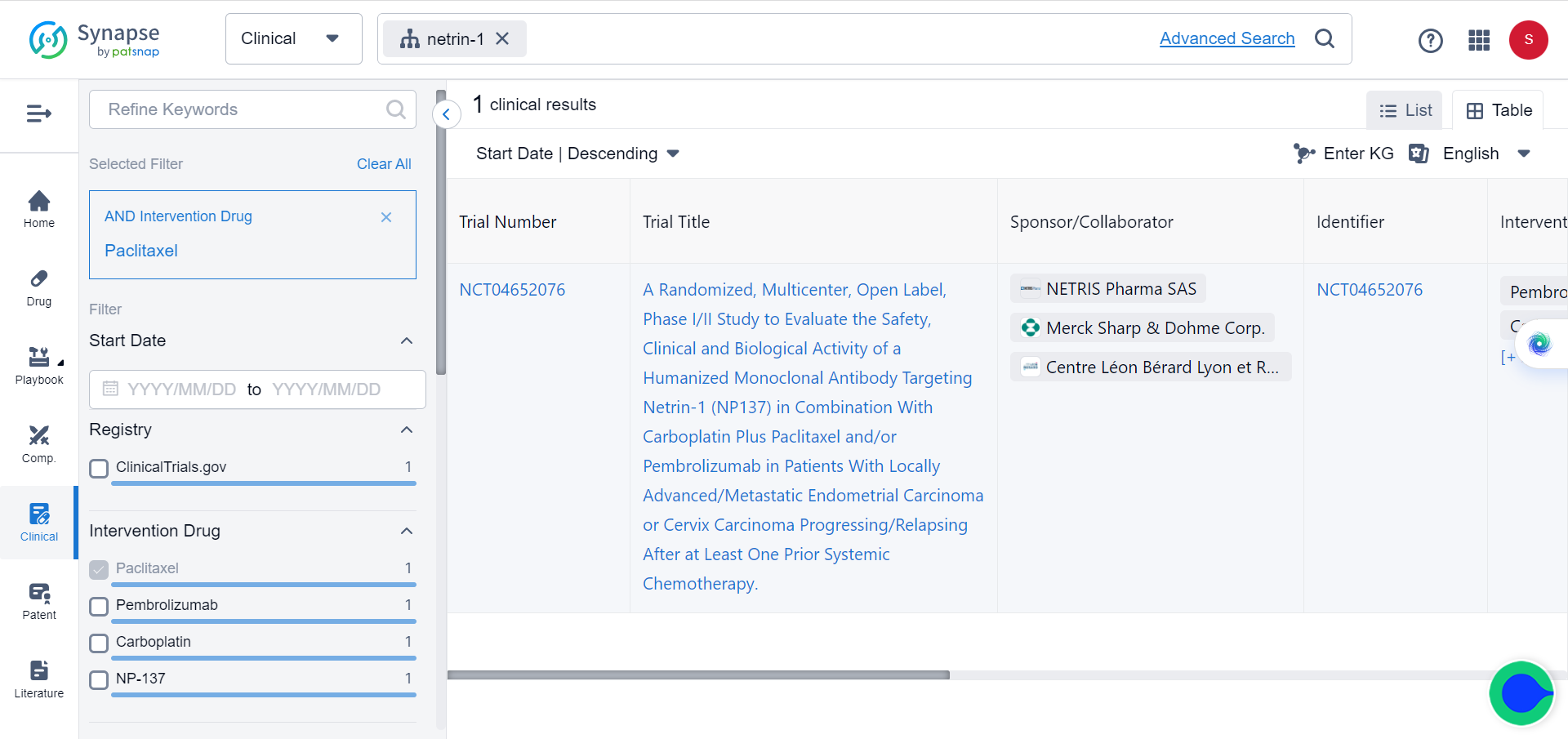
Given the critical role of EMT in conferring resistance to current standard treatments, the researchers further explored the potential of combining NP137 with carboplatin-paclitaxel.
To evaluate the efficacy of combining NP137 with carboplatin-paclitaxel, experiments were conducted in an endometrial cancer (EC) mouse model. They found that the combination treatment resulted in both tumour shrinkage and inhibition of epithelial-to-mesenchymal transition (EMT), which could mitigate resistance to standard therapies. The researchers also performed bulk RNA sequencing, spatial transcriptomics, and single-cell RNA sequencing on matched pre- and on-treatment biopsies from NP137 trial patients with EC, and observed an overall reduction in tumour EMT that was associated with changes in immune infiltrate and increased interactions between cancer cells and the tumour microenvironment. The combination therapy outperformed carboplatin-paclitaxel alone in the EC mouse model, suggesting that NP137 can enhance the effectiveness of standard treatments. These experiments imply that blocking netrin-1 using NP137 in combination with carboplatin-paclitaxel could be a promising therapeutic strategy for treating endometrial cancer.
These findings highlight the clinical significance of Netrin-1 blockade as a dual strategy, addressing both tumor debulking and EMT inhibition. By targeting Netrin-1, researchers believe they have the potential to overcome resistance to standard treatments, offering new hope to patients with endometrial cancer.
This breakthrough study, led by Philippe A. Cassier and a team of international researchers, represents a major advancement in the field of cancer therapeutics. The identification of Netrin-1 blockade as a viable clinical strategy could pave the way for the development of targeted therapies for endometrial cancer and potentially other cancer types characterized by Netrin-1 upregulation.
The research team plans to continue their investigations, conducting further clinical trials to validate the efficacy and safety of NP137 in a larger cohort of endometrial cancer patients. If successful, this approach could revolutionize the treatment landscape for endometrial cancer and potentially impact the broader field of cancer therapy.
As the fight against cancer continues, the discovery of Netrin-1 blockade as a potential therapeutic breakthrough brings renewed hope for patients and clinicians alike, heralding a future where improved treatment options may become a reality.
Reference
Cassier, P.A., Navaridas, R., Bellina, M. et al. Netrin-1 blockade inhibits tumour growth and EMT features in endometrial cancer. Nature (2023).