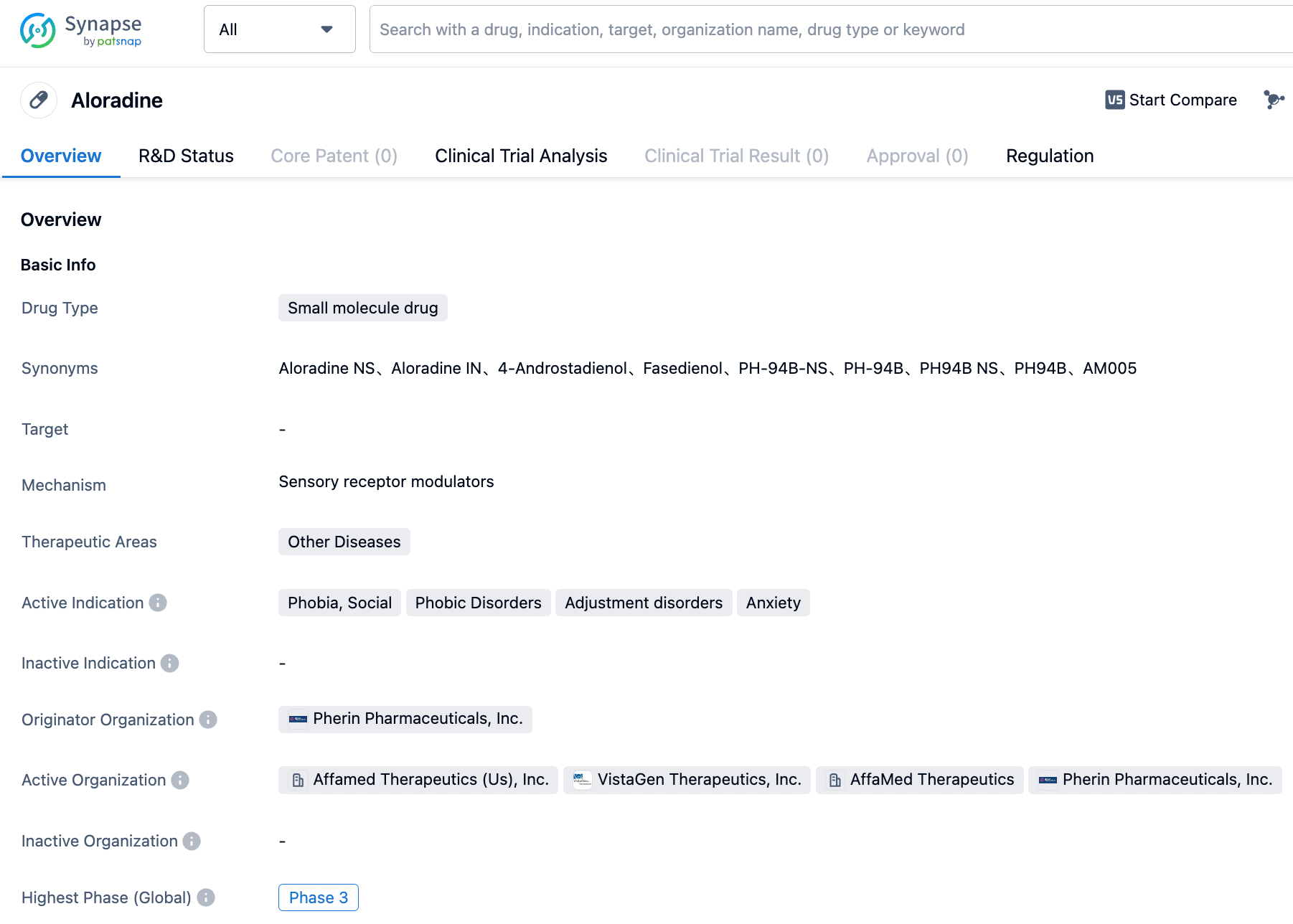
Vistagen's new drug, Fasedienol, reaches Phase 3 clinical trial endpoint, alleviating social anxiety disorder
On August 8, 2023, Vistagen announced the positive top-line results of the PALISADE-2 Phase 3 clinical trial, which assessed the efficacy and safety of its investigational fasedienol (PH94B, Aloradine) nasal spray in adult patients with social anxiety disorder (SAD). The analysis showed that the trial met its primary endpoint, with a statistically significant decrease in average Subjective Units of Distress Scale (SUDS) score during public speaking challenge in fasedienol group patients compared to placebo.
Fasedienol is a fast-acting neurosteroid candidate developed by Vistagen, which can modulate the olfactory-amygdala neural circuit of fear and anxiety, and attenuate the tension of the sympathetic autonomic nervous system, with the potential to treat postpartum anxiety, post-traumatic stress disorder, preoperative anxiety, panic disorder, and other anxiety-related diseases. The drug is administered intranasally in microgram doses, is tasteless, acts quickly (within 15 minutes), and is safe. Unlike existing antidepressants and benzodiazepines, the drug does not directly activate GABA-A receptors, nor does it directly act on the central nervous system of the brain.
ALISADE-2 is a multicenter, random double-blind, placebo-controlled phase 3 clinical trial in adult SAD patients. The trial (n=141) reached its primary efficacy endpoint, with patients receiving fasedienol (n=70) during treatment demonstrating a statistically significantly greater decrease in average SUDS scores (Least Squares [LS] mean = -13.8) compared to placebo (n=71, LS mean = -8.0), with a group difference of -5.8 (p=0.015). The trial also met its secondary endpoint, demonstrating a statistically significant difference between fasedienol and placebo in responder proportions assessed by clinicians using the Clinical Global Impressions-Improvement (CGI-I) scale (p=0.033). The trial showed fasedienol to be well tolerated, with no severe or serious adverse events (AEs). All treatment-emergent adverse events observed during the trial were mild or moderate severity.
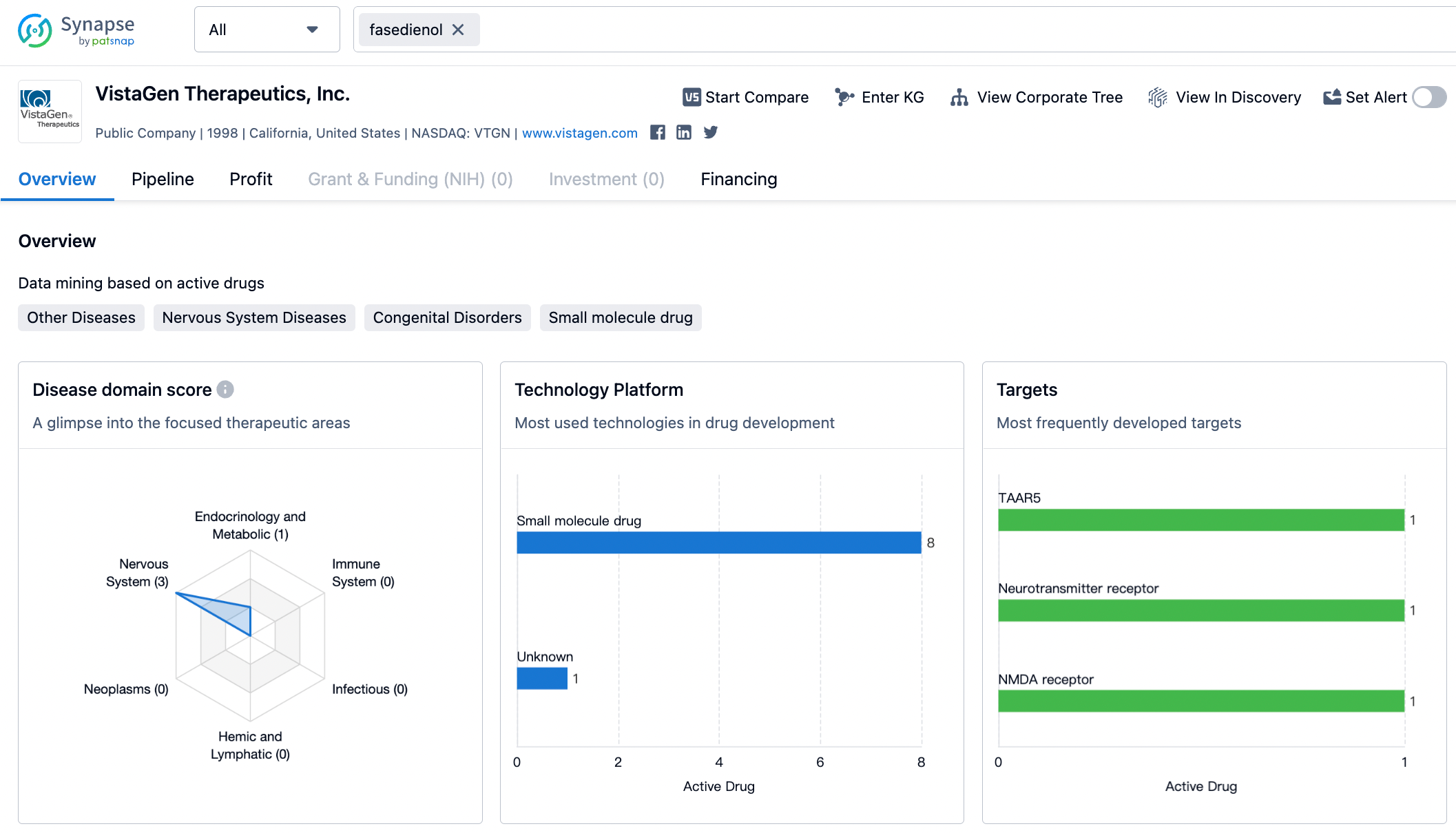
According to the data disclosed by Synapse, as of August 9, 2023, Vistagen has 9 drugs under investigation, and has a very deep lineup in the field of nervous system drugs. Vistagen's new drug fasedienol has a very wide market space, and due to this drug, the company's stock price has surged many times. We look forward to the speedy release of fasedienol to bring new treatment options for "social phobic" patients.