Arrowhead Pharmaceuticals Initiates Phase 1/2a Trial of ARO-CFB for Kidney Diseases Linked to Complement System Dysfunctions
Arrowhead Pharmaceuticals, Inc. has initiated the dosing of initial participants in its Phase 1/2a clinical study of ARO-CFB, its experimental RNA interference therapeutic. This trial is enrolling as many as 66 participants, including both healthy volunteers and individuals diagnosed with complement mediated kidney disease.
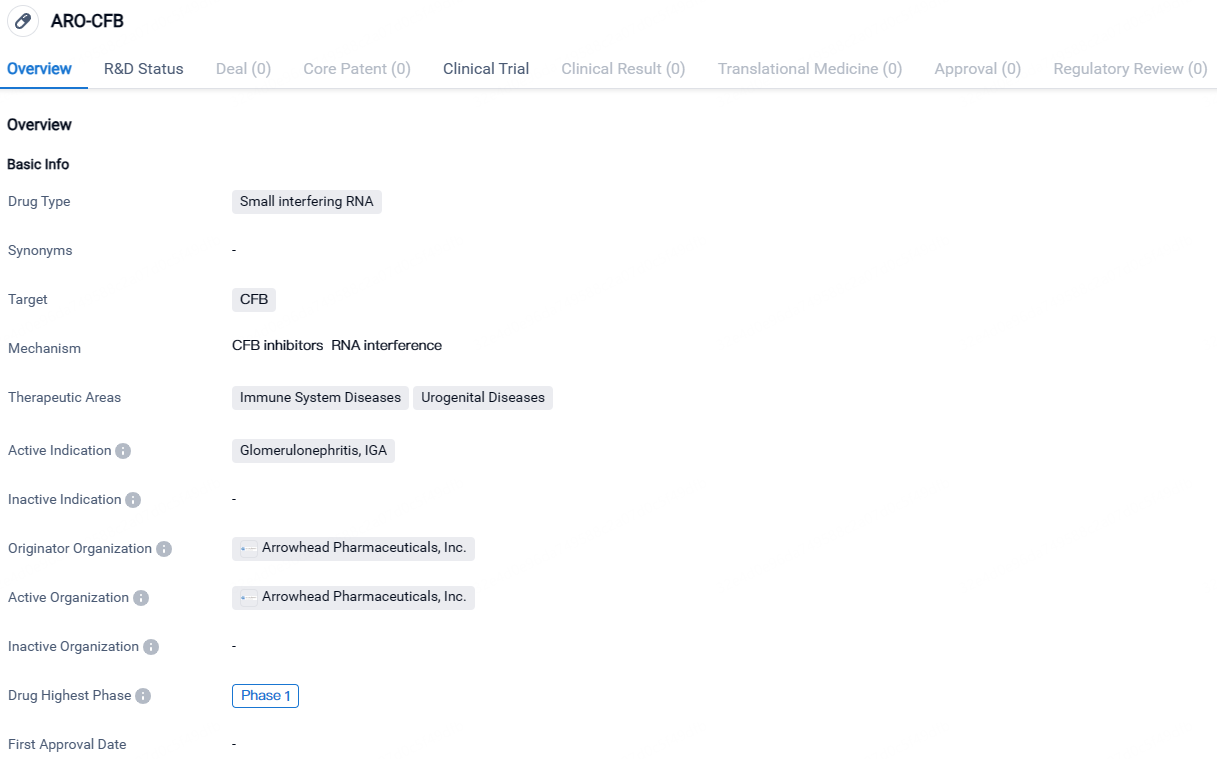
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
In preliminary research studies, ARO-CFB was found to significantly decrease the synthesis of complement factor B in the liver, a key player in triggering the alternative complement pathway which is linked to the etiology of diseases related to complement system activation.
Dr. James Hamilton, who is the Head of Discovery and Translational Medicine at Arrowhead, stated: “ARO-CFB represents our second venture into clinical trials aimed at confronting disorders triggered by the activation of the complement pathway, with our initial endeavor being ARO-C3, a Phase 1 project focusing on complement component 3. We are eager to advance both initiatives to determine if these candidates meet the significant ongoing medical needs in treating various diseases mediated by the complement system.”
ARO-CFB focuses on lowering the liver’s production of complement factor B (CFB), which is crucial in enhancing the activity of the alternative complement pathway and is seen as a viable target for therapy.
Presently under development, ARO-CFB shows potential as a therapy for kidney diseases caused by complement activity, such as immunoglobulin A nephropathy—the most prevalent glomerular disease globally, which significantly risks developing into chronic kidney disease. Moreover, ARO-CFB might also be effective in managing non-renal diseases where complement activation is a factor.
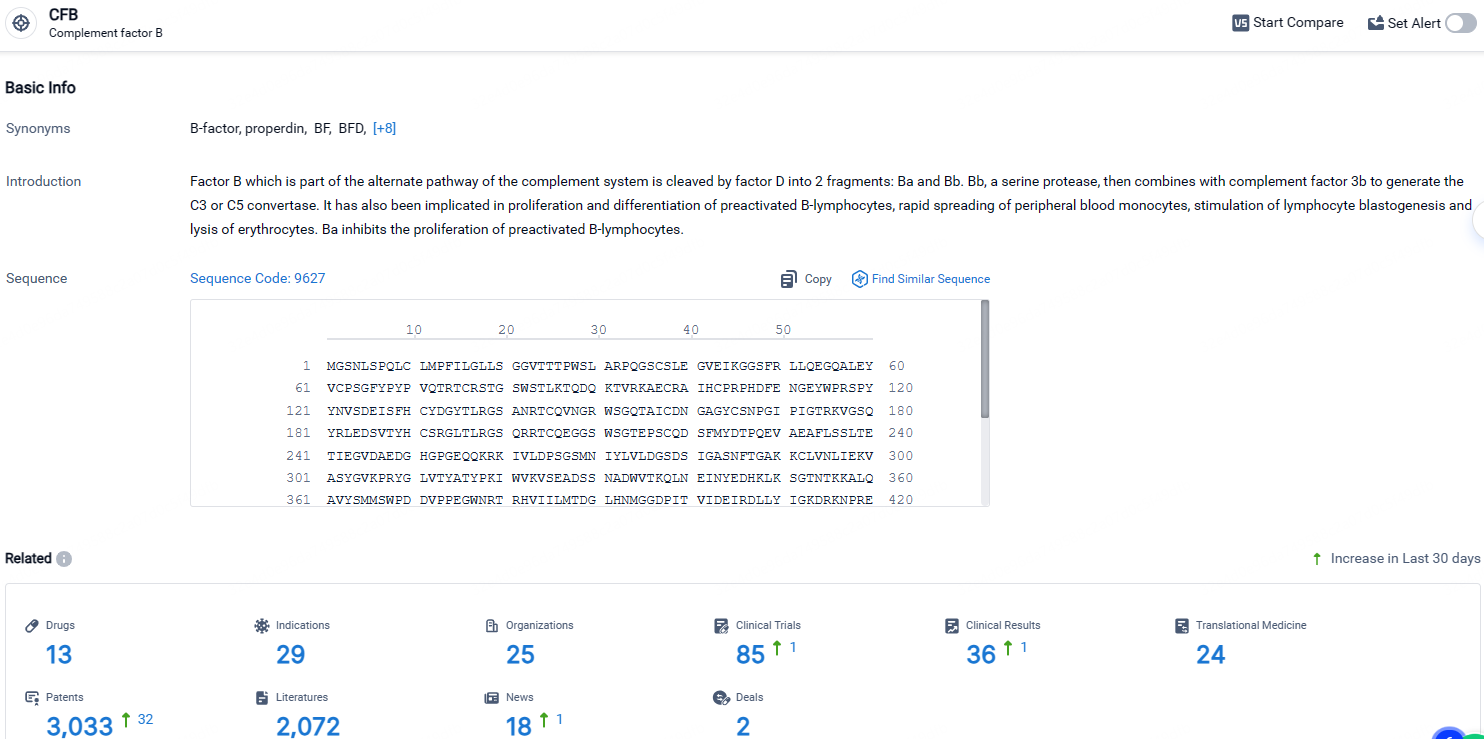
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of April 26, 2024, there are 13 investigational drugs for the CFB target, including 29 indications, 25 R&D institutions involved, with related clinical trials reaching 85, and as many as 3033 patents.
ARO-CFB is a siRNA drug developed by Arrowhead Pharmaceuticals, Inc. that targets the CFB protein. It is being developed for the treatment of immune system diseases and urogenital diseases, with a specific focus on glomerulonephritis, IgA. As the drug is currently in Phase 1 of development, further clinical trials will be needed to assess its efficacy and safety profile.