FDA Approves Novartis's Lutathera® as First Specific Treatment for Young Patients with Gastroenteropancreatic Neuroendocrine Tumors
Novartis has revealed that Lutathera (USAN: lutetium Lu 177 dotatate / INN: lutetium (177Lu) oxodotreotide) has received approval from the U.S. Food and Drug Administration for use in treating children aged 12 and older who have somatostatin receptor-positive (SSTR+) gastroenteropancreatic neuroendocrine tumors, encompassing tumors of the foregut, midgut, and hindgut. This marks the first instance where Lutathera has been specifically sanctioned for treating pediatric patients diagnosed with GEP-NETs.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
"Lutathera has become the inaugural therapy approved for pediatric patients with GEP-NETs, presenting new possibilities for children affected by this uncommon cancer type," stated Tina Deignan, who leads the Oncology division in the US. "The capabilities of radioligand therapies are proving transformative for the oncological landscape. This approval marks a significant milestone in our ongoing efforts to expand the research and application of the RLT platform across a variety of cancers and clinical contexts."
NETs develop from neuroendocrine cells found throughout the body and are typically identified as slow-progressing cancers. Diagnosis is frequently postponed because the symptoms tend to show later, and about 10% to 20% of young patients are identified with the disease at a metastatic stage. Despite being considered a rare condition, the prevalence of NETs has been on the rise over recent years.
"Although GEP-NETs are uncommon in youths, their effects are profoundly distressing. The recent approval fills a deep gap by offering new therapeutic avenues for these young individuals in need," commented Dr. Theodore Laetsch, a trial investigator and the Director of Developmental Therapeutics Program at the Children’s Hospital of Philadelphia, a clinical site for the NETTER-P trial. "Radioligand therapy has revolutionized the treatment landscape for GEP-NETs, and it's promising to see it now extending to benefit the pediatric population."
The decision for approval was supported by findings from the NETTER-P trial, investigating Lutathera in patients aged 12 to less than 18 years diagnosed with SSTR+ GEP-NETs. The trial underscored a safety profile for pediatric patients that aligns with that observed in adults from the NETTER-1 trial — a crucial study that led to Lutathera’s approval for adults. Moreover, the radiation dose received by the pediatric patients was found to be within the safe limits defined for external radiation treatments and was comparable to the levels administered in adults.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
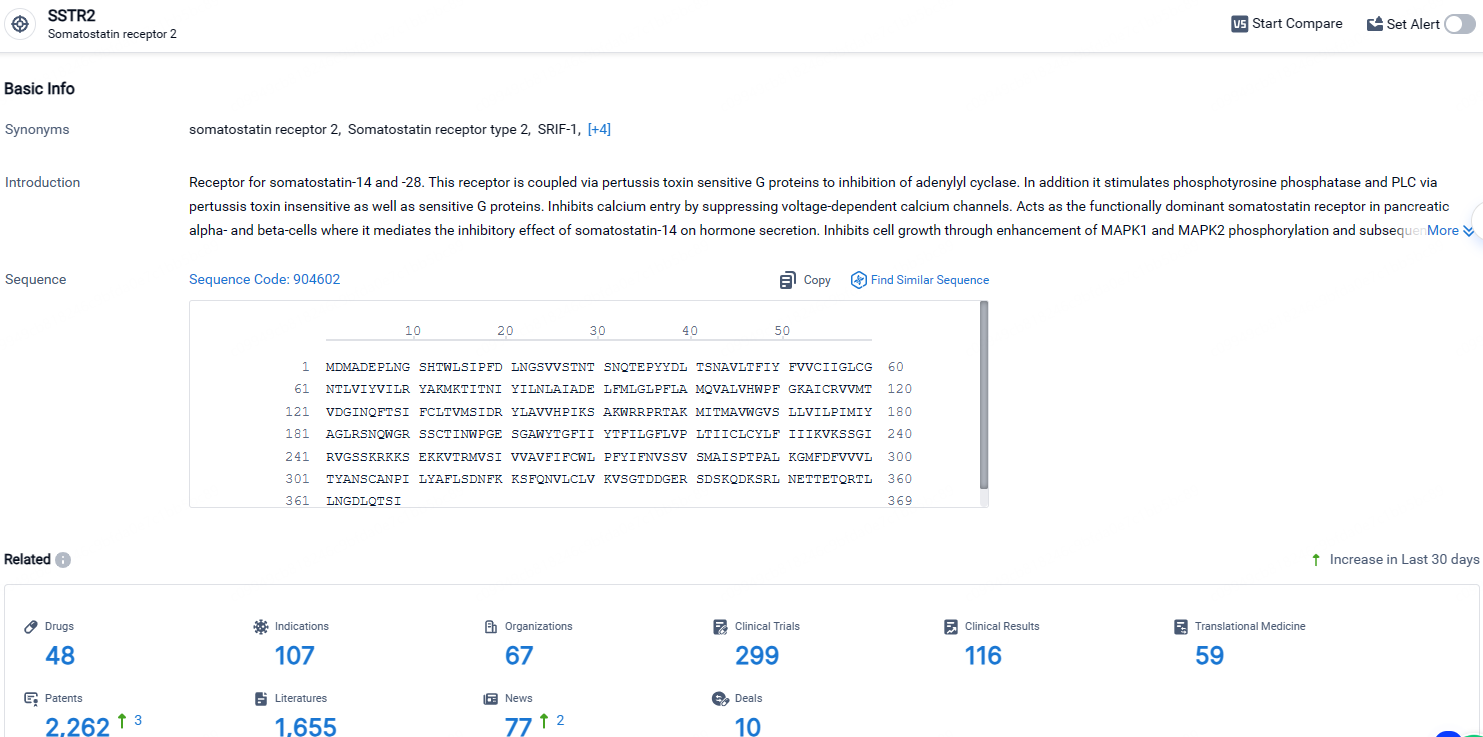
According to the data provided by the Synapse Database, As of April 26, 2024, there are 48 investigational drugs for the SSTR2 targets, including 107 indications, 67 R&D institutions involved, with related clinical trials reaching 299, and as many as 2262 patents.
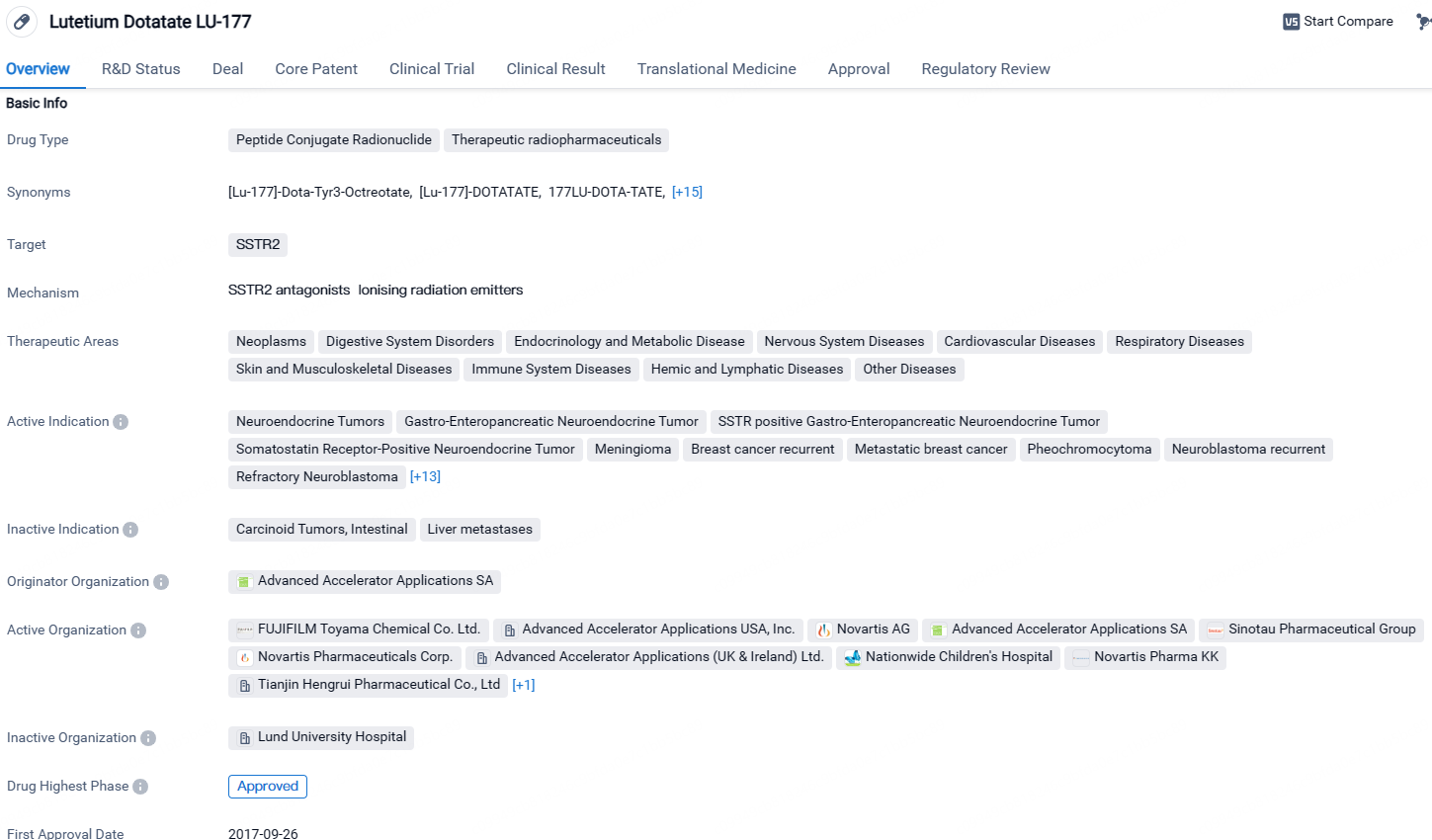
Lutetium Dotatate LU-177 is a peptide conjugate radionuclide drug that targets SSTR2 and has shown promise in treating various diseases across multiple therapeutic areas. Its active indications include a wide range of cancers and neurological conditions. Developed by Advanced Accelerator Applications SA, the drug has received approval in the European Union and other countries, with ongoing clinical trials in China. Its Fast Track status highlights its potential to address unmet medical needs and expedite regulatory processes.