Arrowhead Pharmaceuticals Seeks Approval for Obesity Treatment Trial of ARO-INHBE
Arrowhead Pharmaceuticals, Inc. (NASDAQ: ARWR) revealed that it has submitted an application for regulatory clearance to commence a Phase 1/2a clinical trial of ARO-INHBE. This RNA interference (RNAi) therapeutic is being investigated as a potential treatment for obesity. Additionally, Arrowhead intends to seek regulatory approval by the close of 2024 to launch a clinical trial for its second obesity treatment candidate, ARO-ALK7.
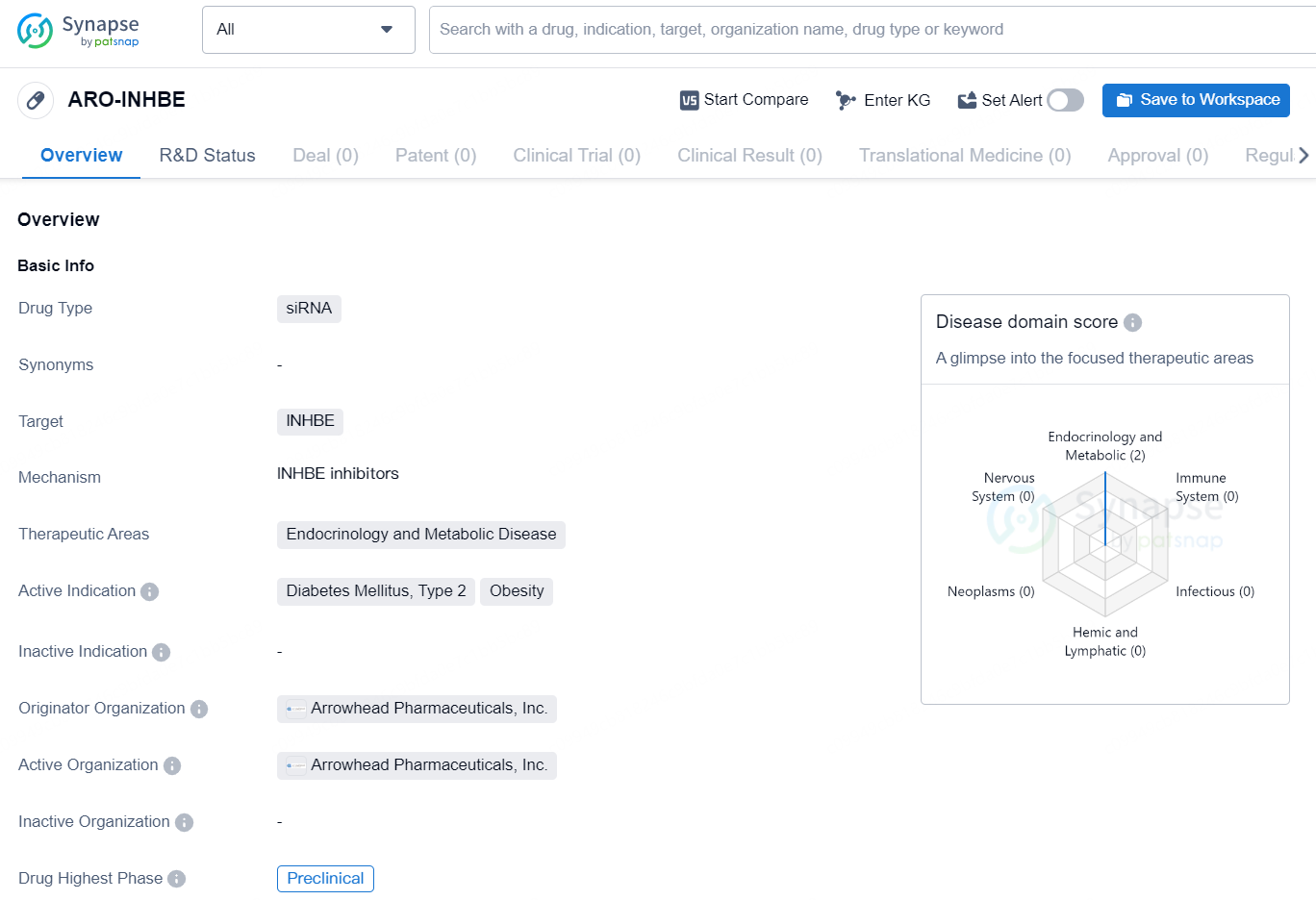
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
Dr. James Hamilton, Chief of Discovery and Translational Medicine at Arrowhead, stated, "Arrowhead’s investigational RNAi therapies for obesity treatment, ARO-INHBE and ARO-ALK7, are crafted to intervene in the pathway that signals fat storage in adipose tissue. Our preclinical data indicates that targeting these pathways reduces body and fat mass while preserving lean muscle mass, thereby enhancing body composition."
The first Phase 1/2 study will assess single and multiple doses of ARO-INHBE monotherapy in obese patients, while the second part will evaluate the combination of ARO-INHBE with tirzepatide in both diabetic and non-diabetic obese patients. Arrowhead believes that innovative therapeutic strategies like ARO-INHBE and ARO-ALK7, which employ novel mechanisms of action, can significantly impact obesity treatment and is eager to begin clinical trials for these promising programs.
ARO-INHBE aims to decrease hepatic expression of the INHBE gene and its product, Activin E. The INHBE target is validated genetically, with loss-of-function INHBE variants in humans associated with reduced obesity and metabolic disease risk, such as type 2 diabetes. Activin E functions as a ligand in a pathway regulating energy balance in adipose tissue. By intervening with investigational ARO-INHBE, there is potential to boost lipolysis, reduce adipose hypertrophy and dysfunction, visceral fat, and insulin resistance.
An application for initiating the clinical trial has been submitted to the New Zealand Medicines and Medical Devices Safety Authority for review by the Standing Committee on Therapeutic Trials. Upon approval, Arrowhead plans to conduct AROINHBE-1001, a Phase 1/2a dose-escalating study focused on evaluating the safety, tolerability, pharmacokinetics, and pharmacodynamics of ARO-INHBE in up to 78 obese adult volunteers. Part 1 will assess single and multiple doses of ARO-INHBE monotherapy, while Part 2 will evaluate ARO-INHBE in combination with tirzepatide. Tirzepatide, a GLP-1/GIP receptor co-agonist administered subcutaneously, has been approved in the US and EU for type 2 diabetes management since 2022, and for weight management starting from 2023/2024.
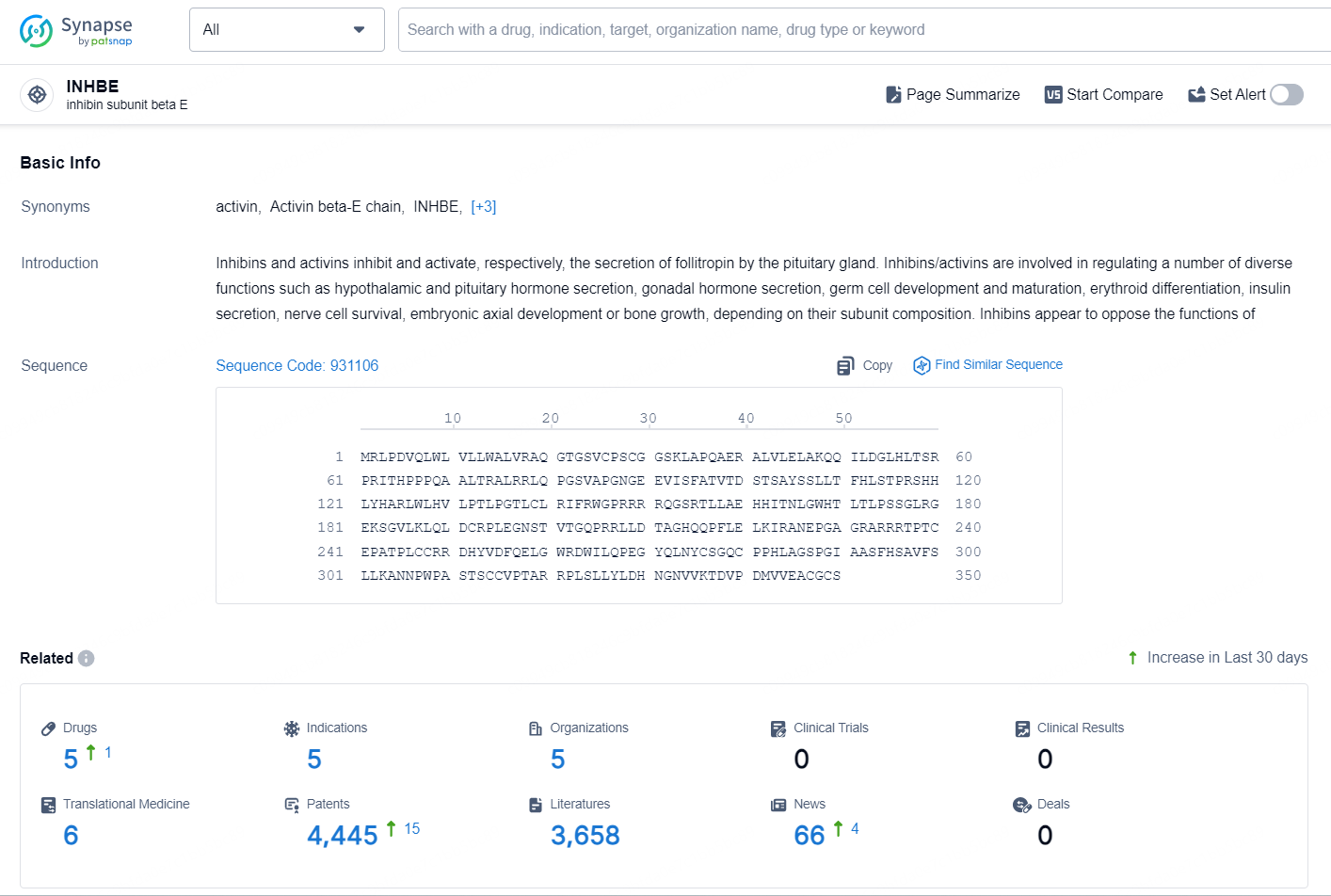
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Chemical, As of September 24, 2024, there are 5 investigational drugs for the INHBE target, including 5 indications, 5 R&D institutions involved, and as many as 4445 patents.
ARO-INHBE is a siRNA drug developed by Arrowhead Pharmaceuticals, Inc. The drug targets INHBE and is focused on therapeutic areas including Endocrinology and Metabolic Disease. Its active indications include Diabetes Mellitus, Type 2 and Obesity. Currently, the drug is in the preclinical stage, with no further information provided on its development status.