AST-201: Aptamer Sciences' Novel Therapy for Liver Cancer in NDA Submission
Aptamer Sciences Inc. declared the submission of an IND (Investigational New Drug) application to the Korean Ministry of Food and Drug Safety to initiate a phase 1 clinical study of their novel compound, AST-201.
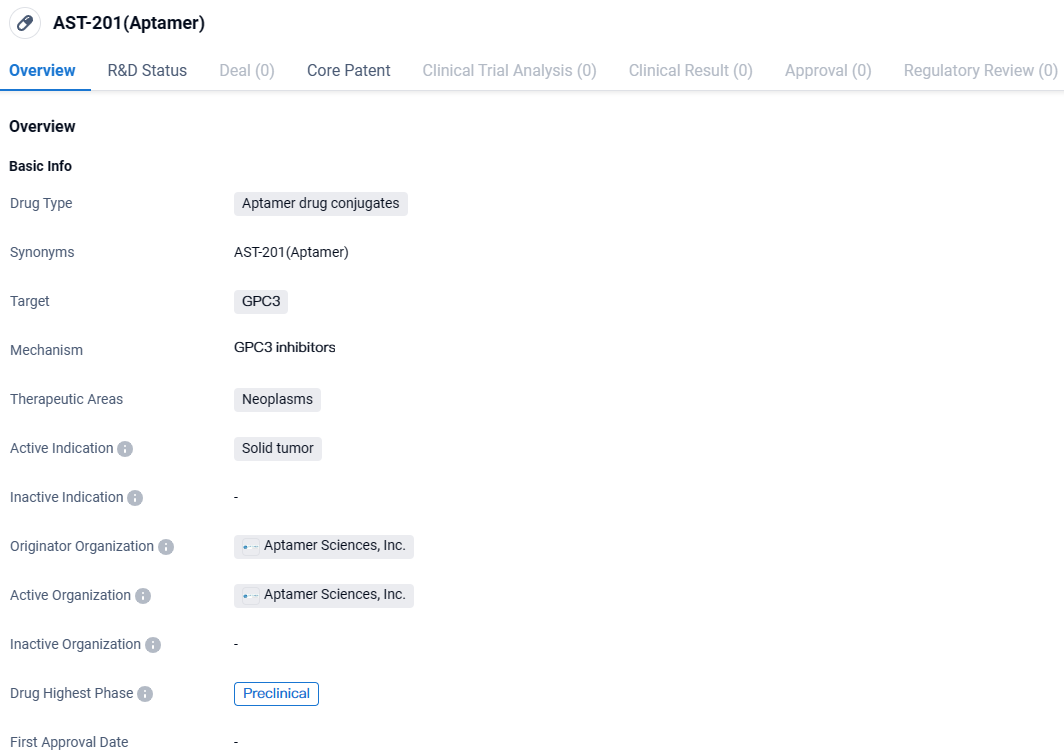
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
The corporation's leading investigational asset, AST-201, stands at the forefront of its development queue, embodying an ApDCTM with a precise action against the GPC3 protein. This target is characteristically overexpressed in particular tumorous tissues, most notably within liver malignancies. The construction of AST-201 integrates the chemotherapeutic agent gemcitabine—acknowledged for its cancer-fighting abilities across a spectrum of malignancies such as ovarian, breast, non-small cell lung, and pancreatic cancers—as its therapeutic cargo. Concurrently, it features an aptamer, meticulously programmed to engage with the GPC3 protein.
Gemcitabine’s choice for the payload is rooted in its balanced toxicity levels, deemed ideal in the context of hepatic therapeutics due to the liver's heightened sensitivity to pharmacological insults. What's more, being a DNA analogue allows gemcitabine to be seamlessly incorporated into the aptamer's nucleotide architecture. Through targeting hepatic carcinoma, the firm aims to bridge the gap presented by the persistent clinical requirements, a commitment underscored by AST-201's promising performance in suppressing tumor growth beyond that of sorafenib within preclinical trials, and its potentiated effects when combined with anti-PD-1 therapy.
Given liver cancer's grave impact, particularly within East Asian regions, where patient outcomes are notoriously bleak, the urgency for more effective treatments is apparent. Despite the adoption of combined regimens like atezolizumab and bevacizumab as contemporary therapeutic norms for progressive liver ailments, revelations from Nature Medicine indicate these may fall short in patient subsets with elevated GPC3 expressions, thus revealing a critical need for more targeted solutions. In this light, AST-201 stands out, pinpointing GPC3 with precision.
The forthcoming phase 1 clinical trial is tailored to scrutinize AST-201’s tolerability, pharmacokinetic properties, and initial anticancer capabilities in individuals bearing advanced, GPC3-positive solid neoplasms. Dr. Hong Jae Chon of the CHA Bundang Medical Center, a key figure in South Korean medical oncology, is appointed to lead the study, which will span across eminent institutions including CHA Bundang Medical Center, Samsung Medical Center, Severance Hospital, and the National Cancer Center.
Dr. Chon has indicated that the therapeutic spotlight is increasingly on GPC3 in the realm of liver cancer R&D within the biopharmaceutical industry. He voiced a positive outlook about AST-201's potential to carve out a niche as an innovative care alternative for those afflicted with liver cancer.
Aptamer Sciences Inc. is committed to demonstrating the clinical virtues of AST-201 and the innovative prowess of its proprietary ApDCTM technology via this study. The firm is also looking forward to this event as a springboard to further its R&D pursuits, thus boosting the market potential of its successive oncological offerings, such as AST-202, which zeroes in on CD25, and other pipeline entities like ApRC, and ApIS.
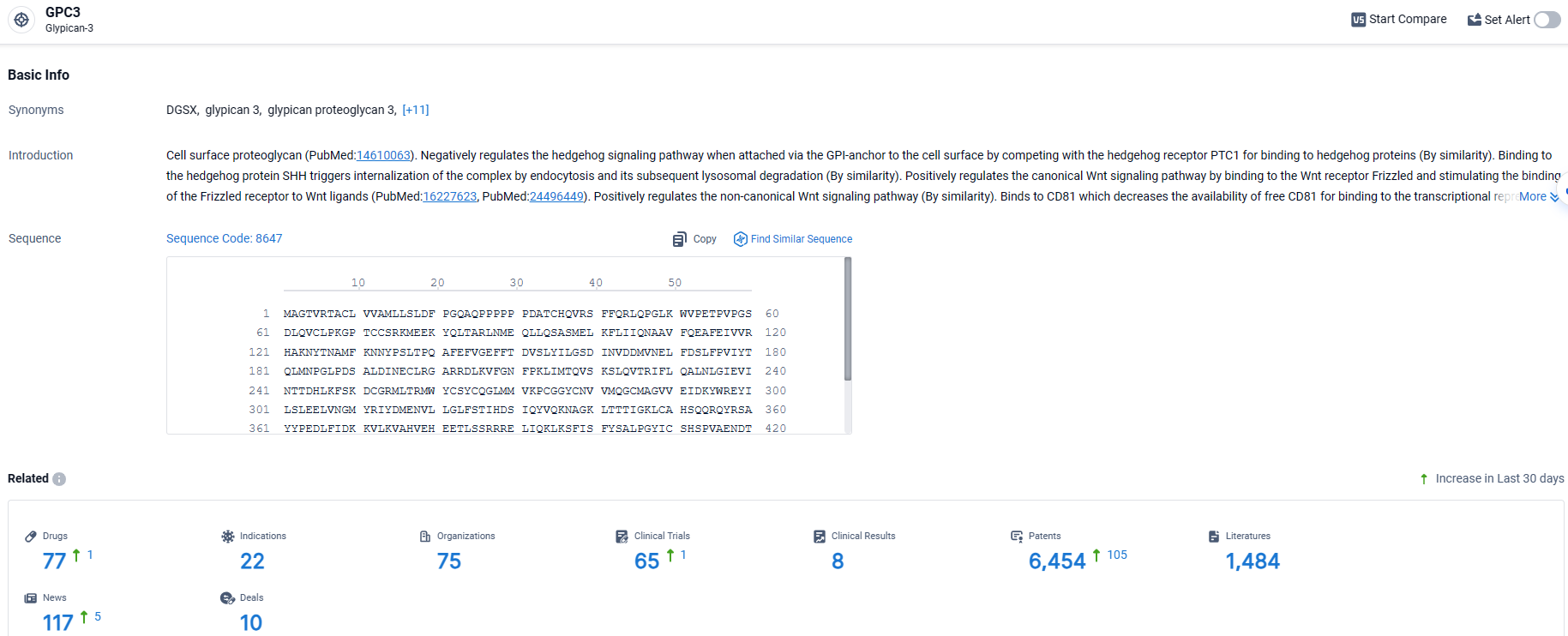
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of March 27, 2024, there are 77 investigational drugs for the GPC3 target, including 22 indications, 75 R&D institutions involved, with related clinical trials reaching 65, and as many as 6454 patents.
AST-201 is an aptamer drug conjugate being developed by Aptamer Sciences, Inc. to target GPC3, a protein commonly found in neoplasms, specifically solid tumors. Currently in the preclinical phase, AST-201 shows potential as a targeted treatment option for solid tumors expressing GPC3, with the aim of improving efficacy and reducing side effects compared to conventional therapies. Further research and development will be necessary to determine its viability as a therapeutic option for patients.