Atara Bio reveals promising initial findings for its off-the-shelf CAR therapy, ATA3431, targeting CD20 and CD19, at ASH's 65th session
Atara Biotherapeutics, Inc., an innovator in the field of T-cell immunotherapy, is utilizing its groundbreaking allogeneic EBV T-cell technology to create impactful treatments for individuals suffering from cancer and autoimmune conditions. The company has recently shared preliminary findings regarding ATA3431, their latest allogeneic therapeutic candidate. This therapy employs a dual-targeted CD20/CD19 chimeric antigen receptor EBV T-cell approach.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Recent evidence underpins the progression of ATA3431 into human trials, with an initial emphasis on therapies for B-cell malignancies. This research will be showcased in a poster session at the 65th annual conference of the American Society of Hematology, scheduled for December 9-12, 2023, in San Diego.
Atara's Executive Vice President and Chief Scientific & Technical Officer, Cokey Nguyen, Ph.D., expressed enthusiasm about ATA3431's trajectory towards an Investigational New Drug (IND) application slated for 2025, noting its robust and promising profile.
Nguyen further remarked on the established efficacy of Atara's EBV T-cell technology, highlighting the treatment of over 500 patients within their program. The strategy for allogeneic EBV T-cell targeting of CD19 is bolstered by extensive academic data showing success with a prior variant of the allogeneic EBV CD19 T-cell product.
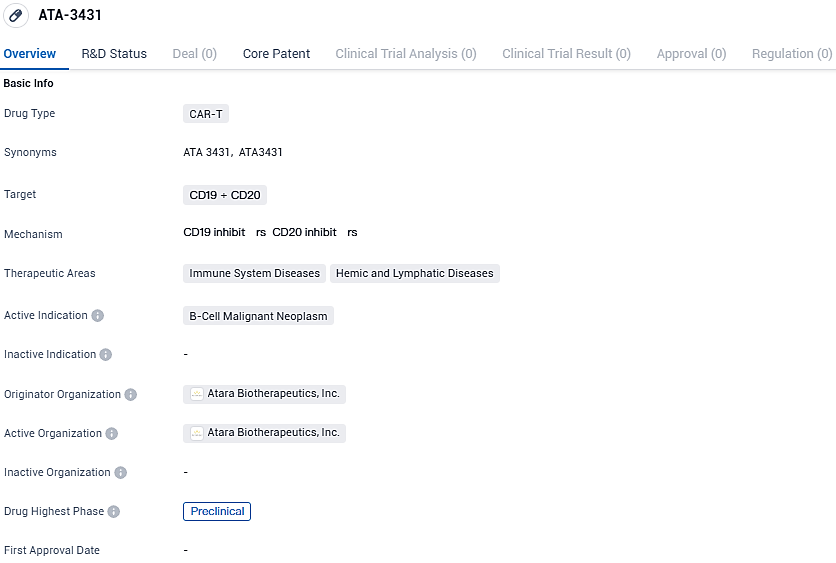
ATA3431 is a dual-targeting, allogeneic CAR that focuses on CD20 and CD19, developed through Atara's proprietary EBV T-cell technology, which bypasses the need for alterations in the T-cell receptor or the HLA gene. The design is a sequential CD20-CD19 arrangement, with binders strategically placed to enhance efficacy. ATA3431 is further reinforced by the inclusion of the 1XX costimulatory domain that has been clinically demonstrated to improve cellular stemness and regulate exhaustion, thus prolonging the functional lifespan of the therapy.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
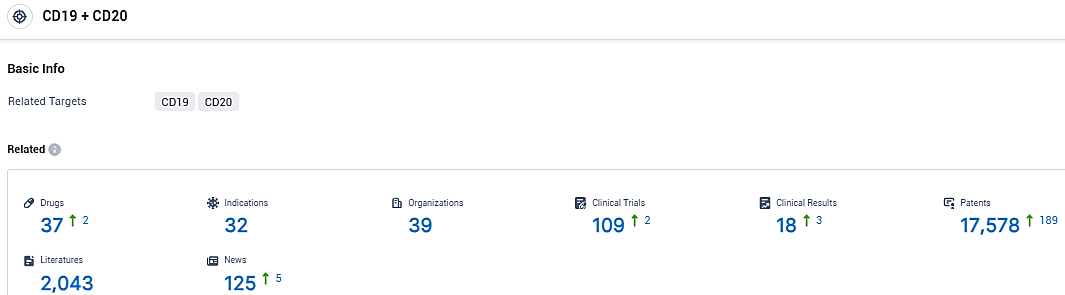
According to the data provided by the Synapse Database, As of December 16, 2023, there are 37 investigational drugs for the CD20/CD19 target, including 32 indications, 39 R&D institutions involved, with related clinical trials reaching 109, and as many as 17578 patents.
ATA-3431 targets CD19 and CD20 proteins found on B-cells and is intended for the treatment of B-cell malignant neoplasms. The drug is currently in the preclinical phase, and its therapeutic areas include immune system diseases and hemic and lymphatic diseases. Atara Biotherapeutics is the originator organization responsible for the development of ATA-3431.