SystImmune collaborates with Bristol Myers Squibb in a major worldwide partnership to advance and market BL-B01D1
SystImmune, an enterprise engaged in clinical-phase biopharmaceutical research, and the pharmaceutical leader Bristol Myers Squibb have declared a unique partnership and licensing deal centered on BL-B01D1, SystImmune's innovative candidate for a leading-edge EGFRxHER3 dual-specificity antibody-drug conjugate. As per the contractual agreement, both entities are committed to collaborating on the advancement and market introduction of BL-B01D1 within the United States.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Via its subsidiary entities, SystImmune is assigned the exclusive duty of advancing, commercializing, and producing within mainland China, along with the obligation to produce select drug inventories for use internationally. Conversely, Bristol Myers Squibb will undertake the exclusive tasks of developing and marketing outside of mainland China.
The bispecific topoisomerase inhibitor-based ADC, BL-B01D1, which targets both EGFR and HER3, is undergoing a comprehensive international Phase 1 clinical trial to assess its safety and therapeutic efficacy in subjects with metastatic or inoperable non-small cell lung cancer.
Earlier in 2023, preliminary clinical results of BL-B01D1 were released at various medical conferences including ASCO, ESMO, and the San Antonio Breast Cancer Symposium. The findings showed encouraging anti-tumor effects in patients with various solid malignancies that had advanced beyond first-line treatments, such as NSCLC and breast cancer.
According to Dr. Yi Zhu, CEO of SystImmune, recent studies involving BL-B01D1 have indicated its substantial effectiveness across a spectrum of solid cancers and a tolerable safety profile. Dr. Zhu expressed admiration for Bristol Myers Squibb's proficiency in global clinical development and the introduction of oncology medicines. Dr. Zhu also noted that the partnership marks a significant progression towards providing promising cancer treatments globally.
Bristol Myers Squibb's Drug Development Chief Medical Officer, Samit Hirawat, MD, EVP, highlighted that integrating SystImmune's BL-B01D1 into their portfolio furthers their strategy of pairing optimal therapies with unaddressed medical needs in solid tumor oncology. Dr. Hirawat looks forward to collaborating with SystImmune to propel BL-B01D1's development, aspiring to introduce a novel therapeutic alternative for those patients who need it most.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
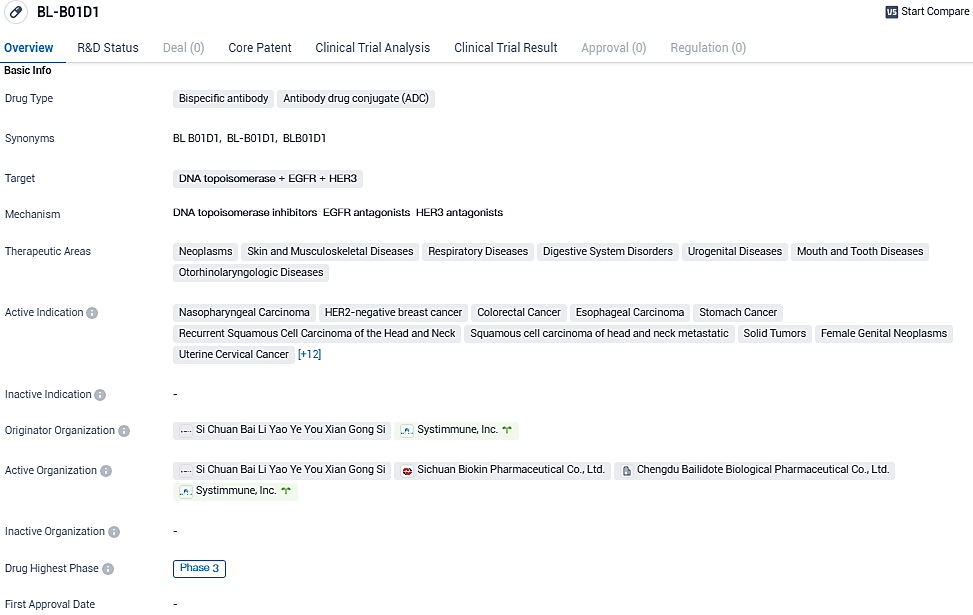
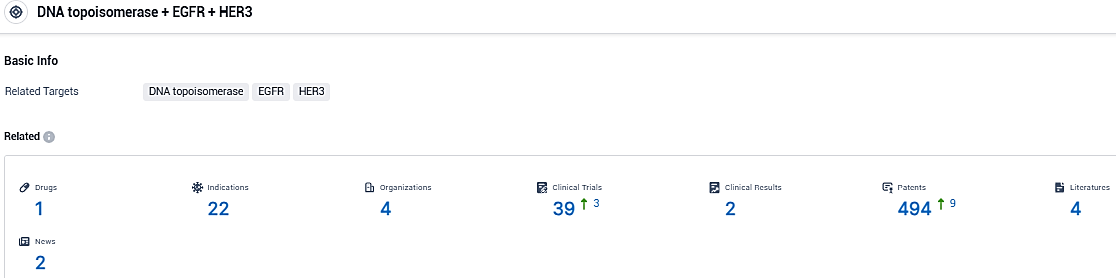
According to the data provided by the Synapse Database, As of December 16, 2023, there are 1 investigational drugs for the DNA topoisomerase and EGFR and HER3 target, including 22 indications, 4 R&D institutions involved, with related clinical trials reaching 39, and as many as 494 patents.
BL-B01D1 is a potentially first-in-class bispecific ADC developed by SystImmune, targeting both EGFR and HER3, which are highly expressed in most epithelial tumors. BL-B01D1 is comprised of SystImmune's proprietary bispecific antibody and linker-payload which contains a stable, cleavable linker and a topoisomerase inhibitor.