Atara Biotherapeutics Submits FDA Application for Tab-cel® to Treat EBV Positive Post-Transplant Lymphoproliferative Disease
Atara Biotherapeutics, Inc., a frontrunner in T-cell immunotherapy, is utilizing its innovative allogeneic Epstein-Barr virus (EBV) T-cell platform to create groundbreaking treatments for cancer and autoimmune disorder patients. The company has announced the submission of a Biologics License Application to the U.S. Food and Drug Administration for tabelecleucel (tab-cel). This application seeks approval for tab-cel as a standalone treatment for adult and pediatric patients aged two years and older suffering from Epstein-Barr virus positive post-transplant lymphoproliferative disease, who have undergone at least one previous therapy.
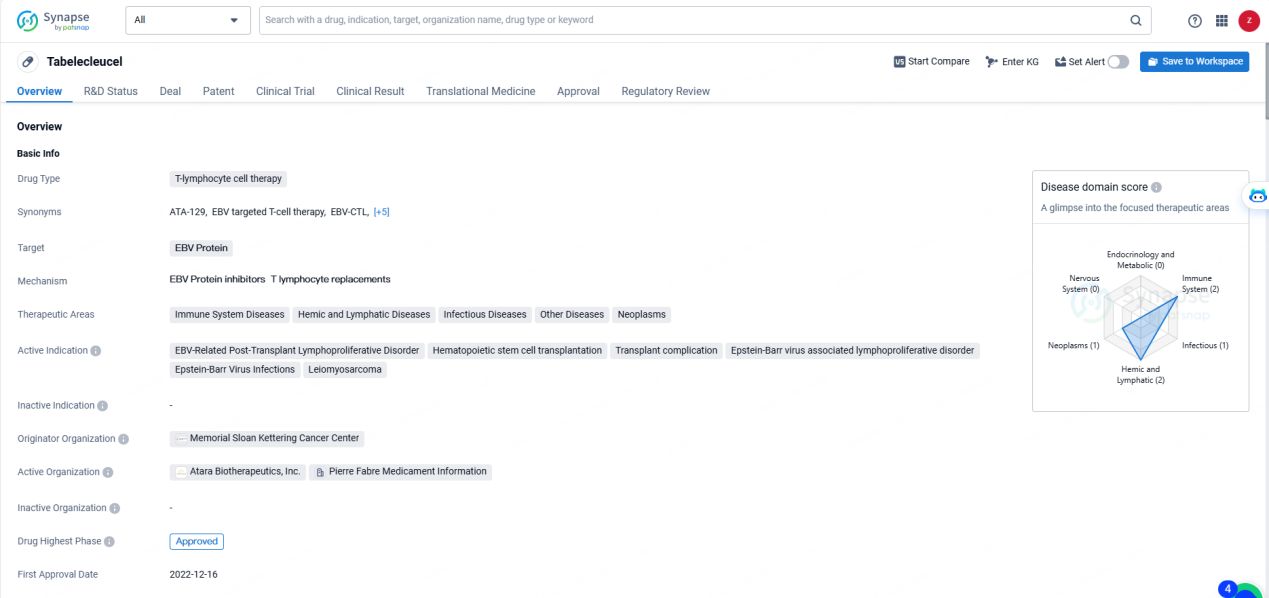
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
"The BLA submission for tab-cel signifies a pivotal moment for Atara, our collaborator Pierre Fabre, and the larger field of allogeneic T-cell therapies, marking an essential stride toward offering this groundbreaking treatment to EBV+ PTLD patients in the U.S.,” stated Pascal Touchon, the President and CEO of Atara. He continued, “My gratitude goes out to the patients and doctors involved in the tab-cel clinical trials, our enduring partners at Memorial Sloan Kettering Cancer Center, and our dedicated internal team for their extraordinary efforts. We eagerly anticipate ongoing collaboration with the FDA during its review and with Pierre Fabre as they prepare for the prospective U.S. launch of this novel therapy.”
Tab-cel, an allogeneic EBV-specific T-cell immunotherapy, aims to identify and destroy EBV-infected cells. The BLA draws on critical and supporting data from over 430 patients treated with tab-cel for various severe conditions, including the recent pivotal ALLELE study, which showcased a statistically significant 48.8% Objective Response Rate and a consistent safety profile with earlier analyses.
The U.S. FDA has awarded Breakthrough Therapy Designation to tab-cel for rituximab-refractory EBV-associated lymphoproliferative disease, as well as orphan drug status.
In December 2023, Atara announced the finalization of an expanded global alliance with Pierre Fabre Laboratories for U.S. and other global markets for tab-cel, extending an initial agreement covering Europe, the Middle East, Africa, and other select emerging markets. Following the BLA submission for tab-cel, Atara progresses the enhanced global partnership with Pierre Fabre, which includes milestone payments of $20 million and $60 million upon successful FDA acceptance and approval of the tab-cel BLA, respectively.
Furthermore, Pierre Fabre is covering Atara's anticipated global development costs for tab-cel until the BLA transfer and is purchasing tab-cel inventory up to the manufacturing transfer date. Atara is also set to receive double-digit tiered royalties on net sales of tab-cel across the U.S. and other designated global markets."
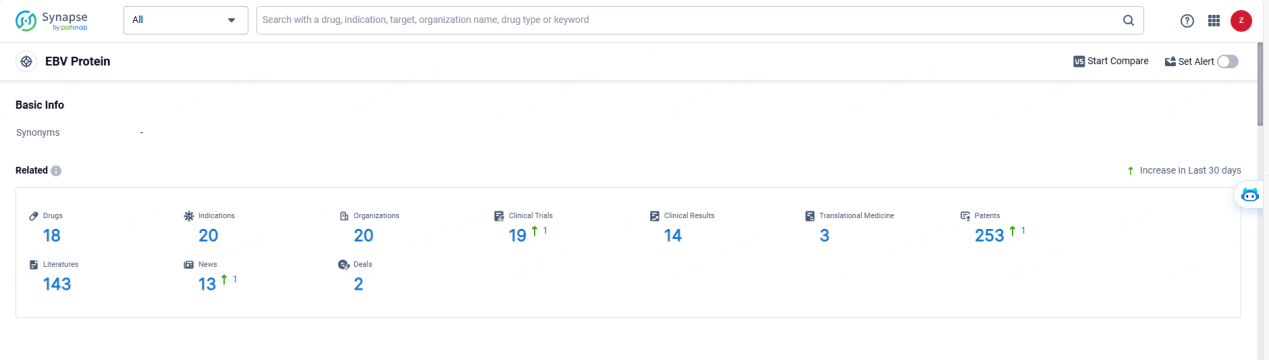
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of May 23, 2024, there are 18 investigational drugs for the EBV target, including 20 indications,20 R&D institutions involved, with related clinical trials reaching 19, and as many as 253 patents.
Tabelecleucel represents a promising development in T-lymphocyte cell therapy and has the potential to significantly impact the treatment landscape for a range of diseases, particularly those related to EBV infections and lymphoproliferative disorders. Its approval in multiple countries and the regulatory designations it has received underscore its importance in addressing critical medical conditions. As such, Tabelecleucel is poised to make a meaningful contribution to the pharmaceutical industry and the broader healthcare sector.