Atezolizumab: A Quick Look at Its R&D Progress and Clinical Results from the 2024 AACR
Treatment (tx) for advanced (LA) squamous cell carcinoma of the head and neck (SCCHN) includes a combination of surgery, radiation and/or chemotherapy followed by monitoring for local recurrence/distant metastases as standard of care. Given poor outcomes, there remains a clear unmet need in this pt population. On April 5, 2024, the latest clinical trial of Atezolizumab (atezo) after definitive local therapy vs placebo in patients (pts) with high-risk LA SCCHN were reported in 2024 AACR.
Atezolizumab's R&D Progress
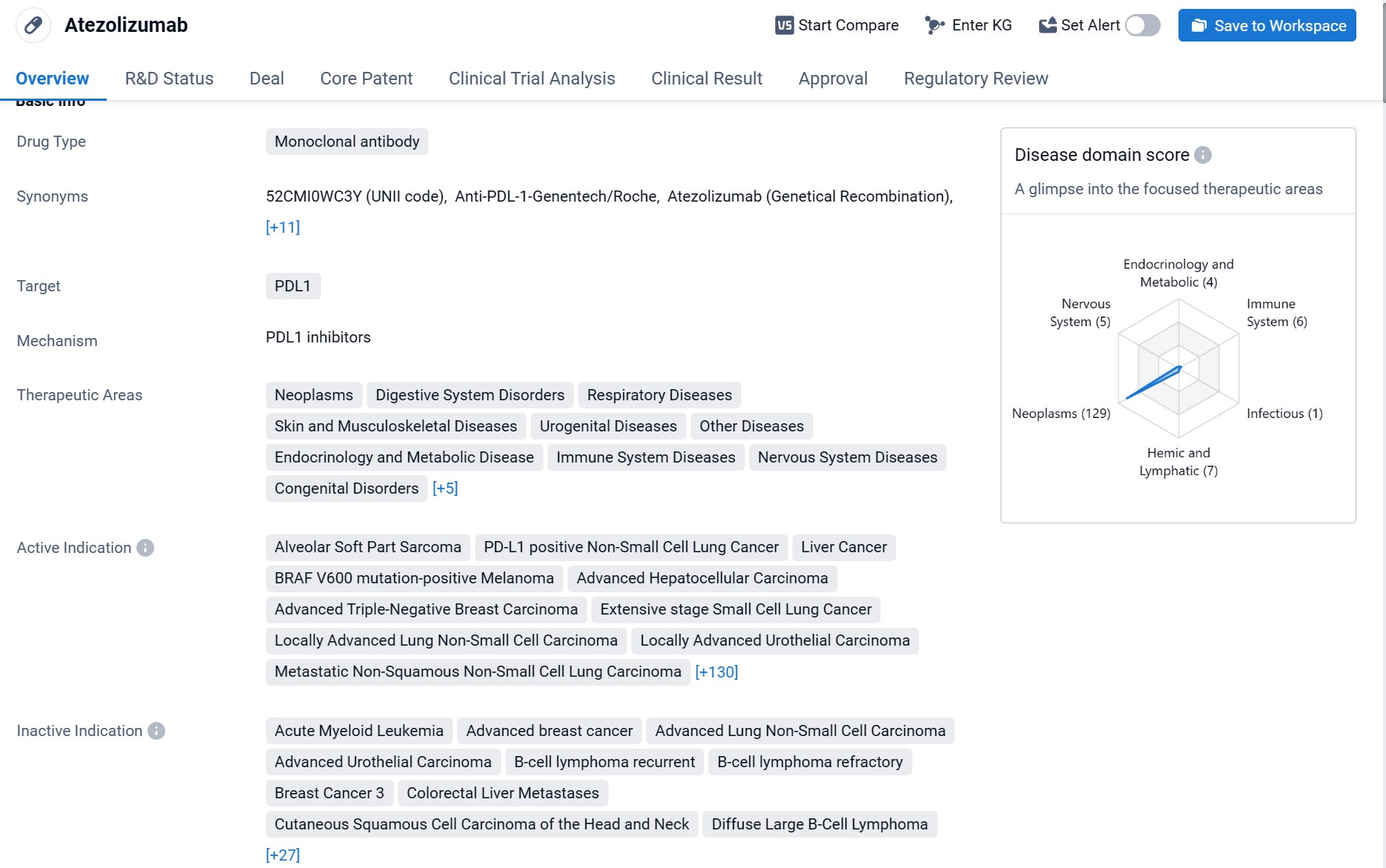
Atezolizumab is a monoclonal antibody drug that targets PDL1 and is used in the treatment of various diseases. The drug is primarily used in the field of biomedicine and has shown efficacy in treating neoplasms, digestive system disorders, respiratory diseases, skin and musculoskeletal diseases, urogenital diseases, and other diseases.
According to the Patsnap Synapse, Atezolizumab has been approved for use in the United States and China. And the clinical trial distributions for Atezolizumab are primarily in the United States, China and United Kingdom. The key indication is Neoplasms.
Detailed Clinical Result of Atezolizumab
This randomized, parallel assignment, quadrupled-masked phase 3 study (NCT03452137) was aimed to evaluate the efficacy and safety of atezo in pts with LA SCCHN who are at high-risk for disease progression following multi-modal definitive tx.
In this study, eligible pts with LA SCCHN (Stage IVa or IVb involving the oral cavity, larynx, hypopharynx, HPV negative oropharynx or Stage III HPV positive oropharynx [per AJCC 8th edition]) with no disease progression after multi-modal definitive tx were randomized (1:1) to receive atezo 1200 mg or placebo. Tx was given every 3 weeks for 1 year or until disease progression, unacceptable toxicity or consent withdrawal. Primary endpoint: investigator-assessed event-free survival (INV-EFS). Other key endpoints: overall survival (OS, secondary for efficacy) and safety.

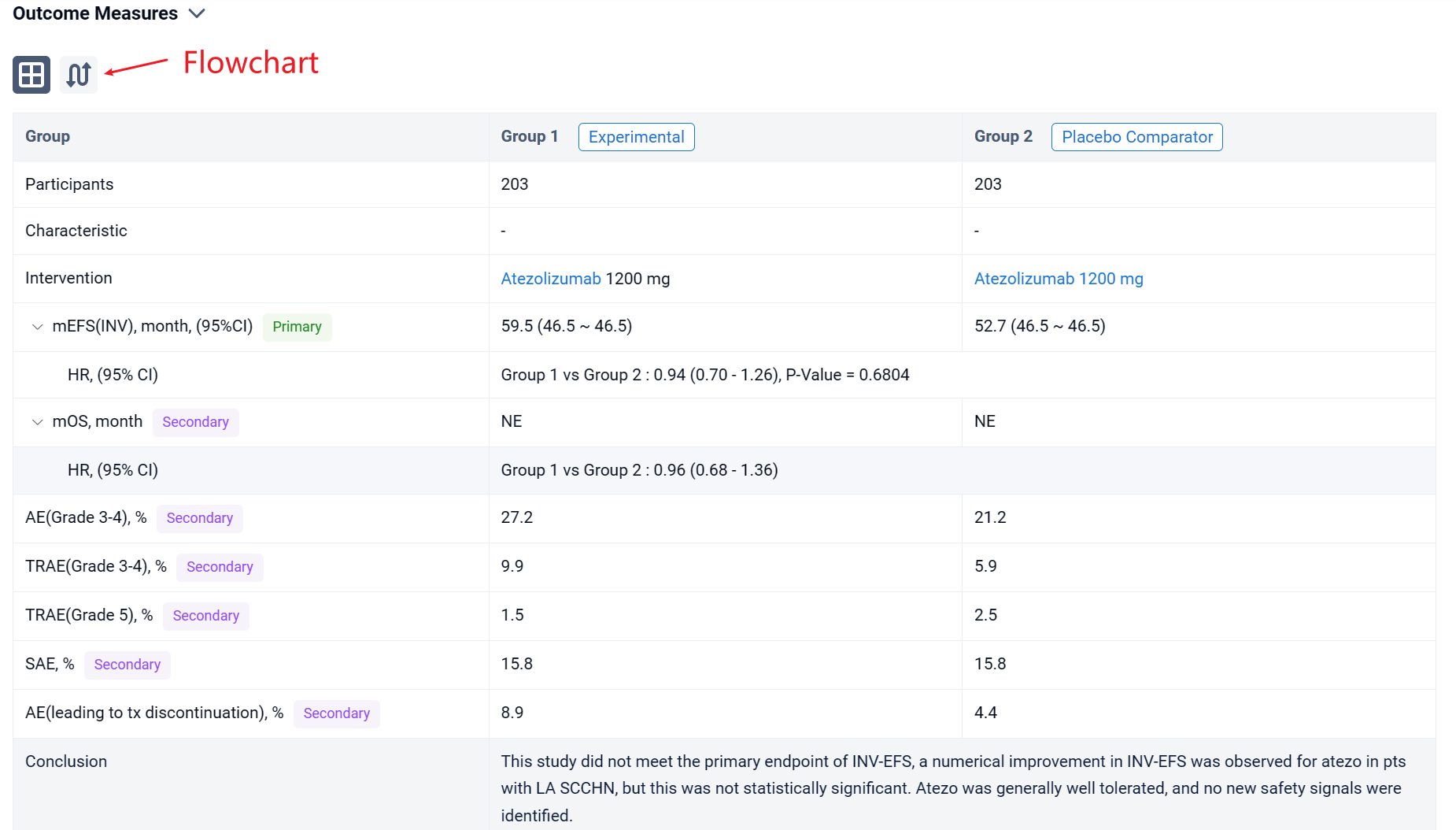
The result showed that a total of 406 pts were randomized to receive either atezo (n=203) or placebo (n=203). 156 (38.4%) pts underwent surgery as part of definitive tx. At clinical cutoff (27 September 2023), median follow-up was 46.5 months (mos). Median INV-EFS was 59.5 mos with atezo vs 52.7 mos with placebo (HR, 0.94; 95% CI, 0.70-1.26). INV-EFS results were generally consistent across all subgroups. OS showed no difference between arms for atezo vs placebo.
It can be concluded that this study did not meet the primary endpoint of INV-EFS, a numerical improvement in INV-EFS was observed for atezo in pts with LA SCCHN, but this was not statistically significant. Atezo was generally well tolerated, and no new safety signals were identified.
How to Easily View the Clinical Results Using Synapse Database?
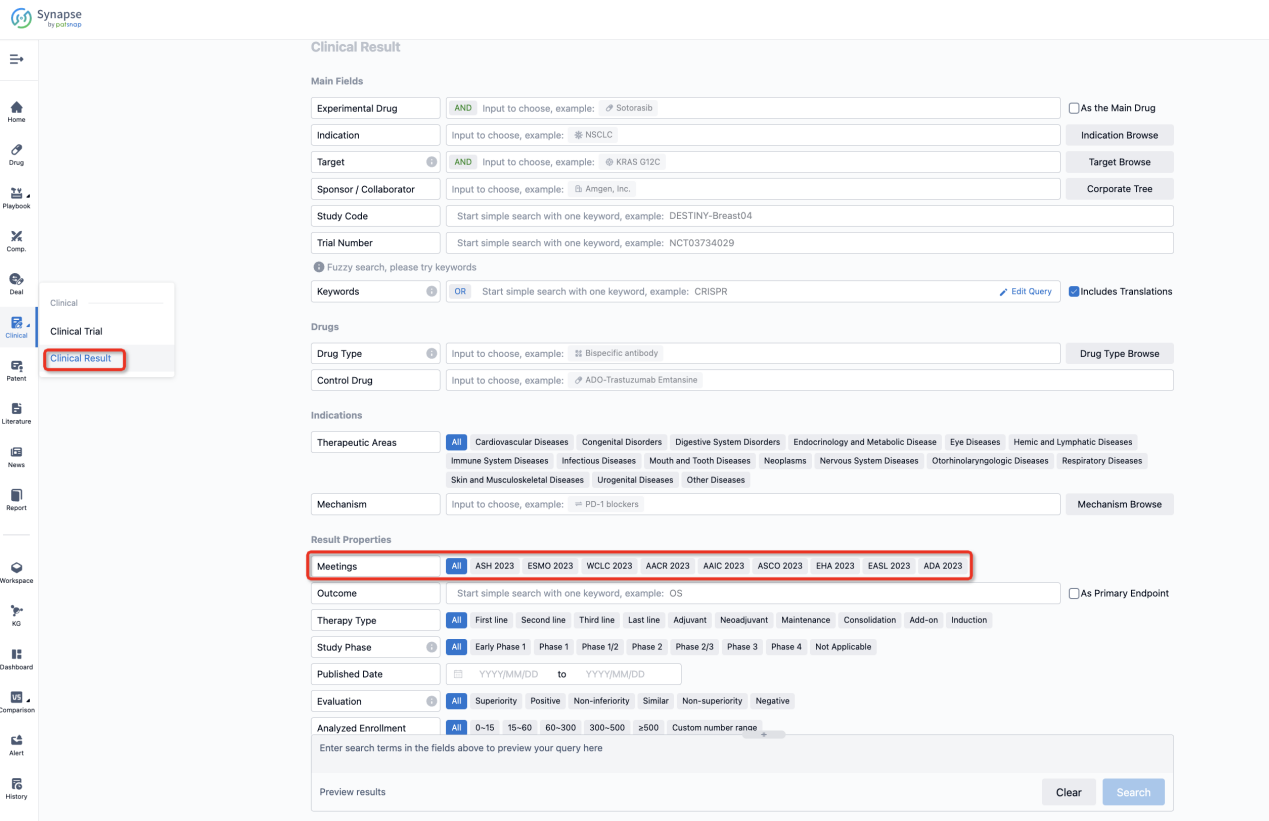
If you want to know the other clinical results of popular conferences, please lick on the “Clinical Results” on the homepage of Patsnap Synapse, which provides multi-dimensional screening and filtering of drugs, indications, targets, companies, result evaluation, release date, popular conferences, etc. to help you quickly locate the data you need.
Select the clinical meeting you are interested in, such as ESMO. In the results, you can quickly locate the data you want to view by indication, phase and drug name.
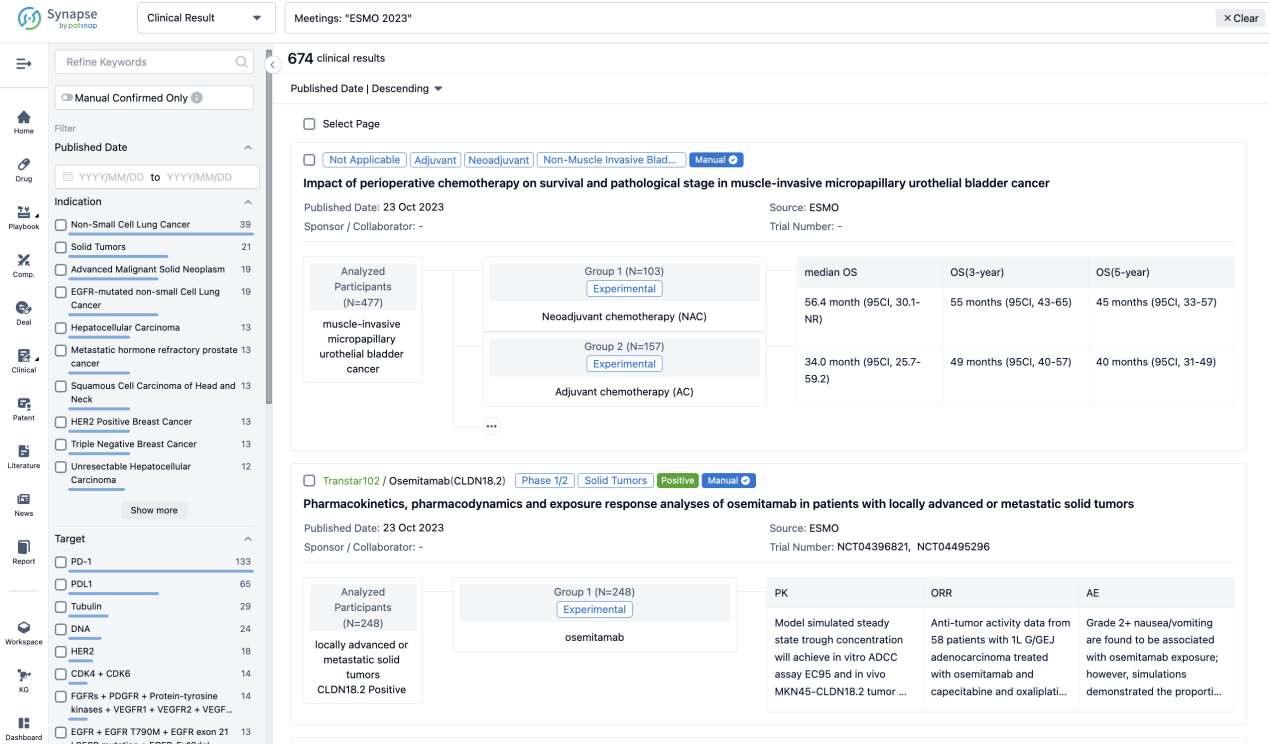
A single result clearly shows important information such as registration number, phase, indication, Sponsor/Collaborator, biomarker, Trial number, dosing regimen and more.
If you would like to view more information about this result, you can go to the result detail page by clicking on the title.
Above the headings, we provide the original source of the outcome data. The basic information is supplemented with more information beyond the list, such as company, study. design, etc.

In the important Outcome Measures section, we provide both list and flowchart forms, which are convenient for you to overview the comparison group information and core indicator data.

Finally, if you need to download these results, you can conveniently check the check boxes on the left side of the list, or directly click the "Export" button to download the data for personalized analysis and file sharing.
Click on the image below to embark on a brand new journey of drug discovery!