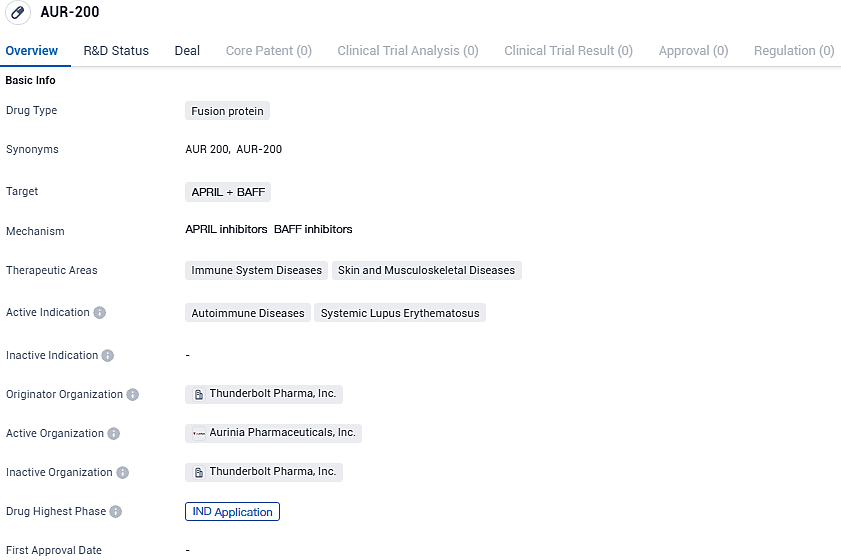
Aurinia Pharmaceuticals has formally submitted an IND application for its drug candidate AUR 200 to the FDA
Aurinia Pharmaceuticals Inc. declared that it has lodged its Investigational New Drug application with the U.S. Food and Drug Administration, targeting approval to proceed with the development of their new therapeutic agent AUR200. This novel treatment represents an advanced candidate for tackling autoimmune disorders linked to B-cell activity.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Following authorization from the FDA, Aurinia is scheduled to launch a preliminary Phase 1 clinical trial during the upcoming first half of 2024. This trial aims to ascertain the safety, tolerability, and pharmacological characteristics, including pharmacokinetics and pharmacodynamics, of AUR200 through a study involving healthy test subjects.
AUR200 is characterized as a highly effective, targeted Fc-fusion protein that includes a tailored B cell maturation antigen designed for enhanced attachment to BAFF (B-cell Activating Factor) and APRIL (A Proliferation-Inducing Ligand). The proteins BAFF and APRIL are crucial in the management of B-cell proliferation and survival.
Volker Knappertz, M.D., Executive Vice-President of Research & Development at Aurinia, expressed optimism about the progression of AUR200 within Aurinia’s product line and the broader vision of transforming treatments in autoimmune diseases. "The submission of our IND for AUR200 signifies a significant progression for Aurinia's development slate. With the promising nature of AUR200's profile and its preclinical efficacy, we anxiously await the opportunity to disclose subsequent findings and updates as we embark on our clinical trial endeavors," he commented.
The effectiveness of AUR200 was illustrated through preclinical results showcased at the American College of Rheumatology Convergence 2022. In the preclinical trial using a lupus mouse model, therapeutic dosing of AUR200 led to a reduction in numerous biomarkers associated with disease activity and notably improved survival rates.
In addition to the positive impacts seen in mice, AUR200 was also found to be well-received in cynomolgus monkeys without eliciting negative side effects, underscoring the therapeutic promise of AUR200 in the management of autoimmune conditions.
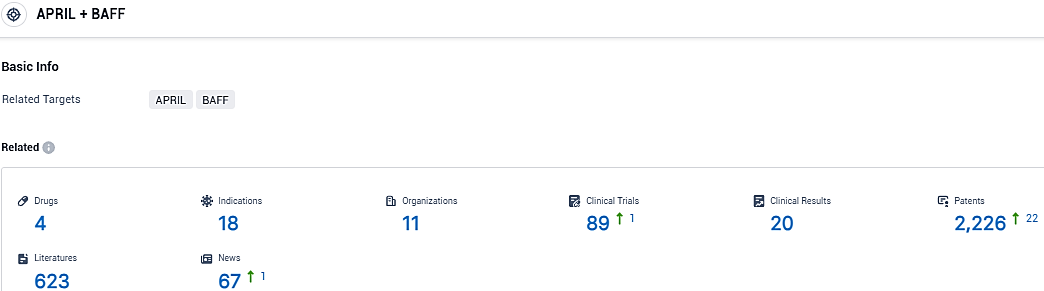
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of December 28, 2023, there are 4 investigational drugs for the APRIL and BAFF target, including 18 indications, 11 R&D institutions involved, with related clinical trials reaching 89, and as many as 2226 patents.
AUR-200 targets APRIL and BAFF and is primarily intended for the treatment of autoimmune diseases, specifically systemic lupus erythematosus. The drug has potential applications in immune system diseases, as well as skin and musculoskeletal diseases. Currently, AUR-200 is in the IND application phase, representing an important step towards clinical trials and potential future availability for patients.