Merck Announces an Update on the U.S. Regulation Process for Gefapixant
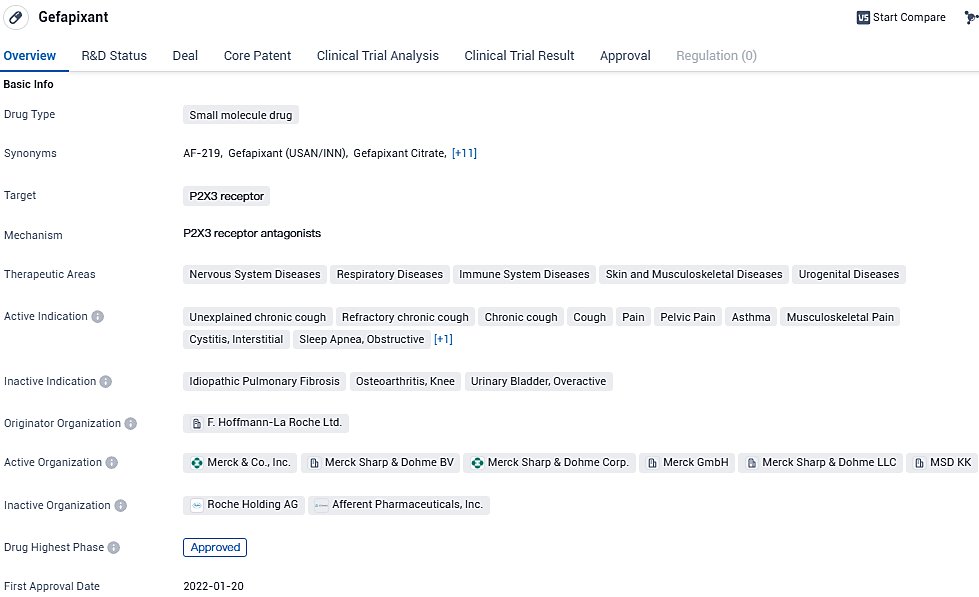
The pharmaceutical giant Merck, which operates under the name MSD in regions outside the U.S. and Canada, recently disclosed that it received a Complete Response Letter from the U.S. Food and Drug Administration (FDA) pertaining to the company's submission of a New Drug Application for the drug gefapixant. Gefapixant, designed for oral administration, is a non-opioid, selective antagonist of the P2X3 receptor. It is currently undergoing testing for its potential to alleviate persistent chronic coughs, or those chronic coughs with no clear cause, in adult patients.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
In the Complete Response Letter (CRL), the Food and Drug Administration (FDA) determined that the evidence provided by Merck was not adequately convincing to demonstrate the drug's efficacy in the management of Renal Cell Carcinoma (RCC) and Unexplained Chronic Cough (UCC). The CRL's concerns were not about the safety profile of gefapixant. Merck is currently assessing the commentary provided by the FDA to figure out the forthcoming actions.
A cough that persists for more than eight weeks is characterized as chronic. Renal Cell Carcinoma (RCC)-related chronic cough continues in adults even after standard therapy for comorbidities like asthma or GERD has been administered, while a UCC is identified when no concrete cause is discovered after exhaustive diagnostic efforts.
Dr. Joerg Koglin, Merck Research Laboratories' senior vice president of global clinical development, expressed appreciation to the participants and researchers of clinical trials for their vital role in expanding the understanding of chronic cough. They contribute significantly to cognizance around the substantial healthcare need and the effects of RCC and UCC on those afflicted.
Dr. Koglin voiced dissatisfaction regarding the lack of sanctioned treatment options for resistant or indeterminate chronic cough, in light of the FDA's rejection of the new drug application for gefapixant.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
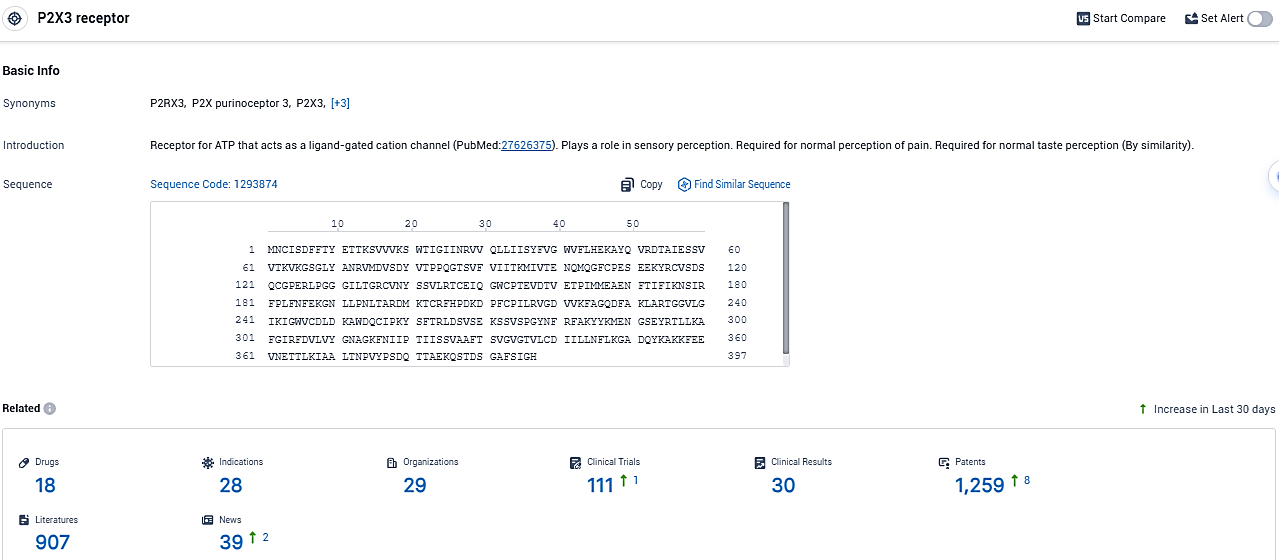
According to the data provided by the Synapse Database, As of December 28, 2023, there are 18 investigational drugs for the P2X3 target, including 28 indications, 29 R&D institutions involved, with related clinical trials reaching 111, and as many as 1259 patents.
As a small molecule drug targeting the P2X3 receptor, Gefapixant holds promise in addressing various diseases and symptoms related to the nervous system, respiratory system, immune system, skin, musculoskeletal system, and urogenital system. Chronic cough, pain, and asthma are among the conditions that could potentially benefit from this drug. The approval of Gefapixant highlights the potential efficacy and safety of the drug.