Azitra, Inc. Presents Promising ATR-12 Data and Netherton Syndrome Plans at ASGCT Conference
Azitra, Inc., a biopharmaceutical company in the clinical stage dedicated to creating cutting-edge therapies in precision dermatology, has released preclinical data from its platform and pipeline. These findings will be presented on Friday, May 10, 2024, during two oral sessions titled “Engineered Staphylococcus Epidermidis as a Protein Delivery System for Treating Skin Diseases” and “Staphylococcus epidermidis Strain Expressing LEKTI-D6 for Netherton Syndrome” at the American Society of Gene and Cell Therapy 2024 Annual Meeting in Baltimore, MD.
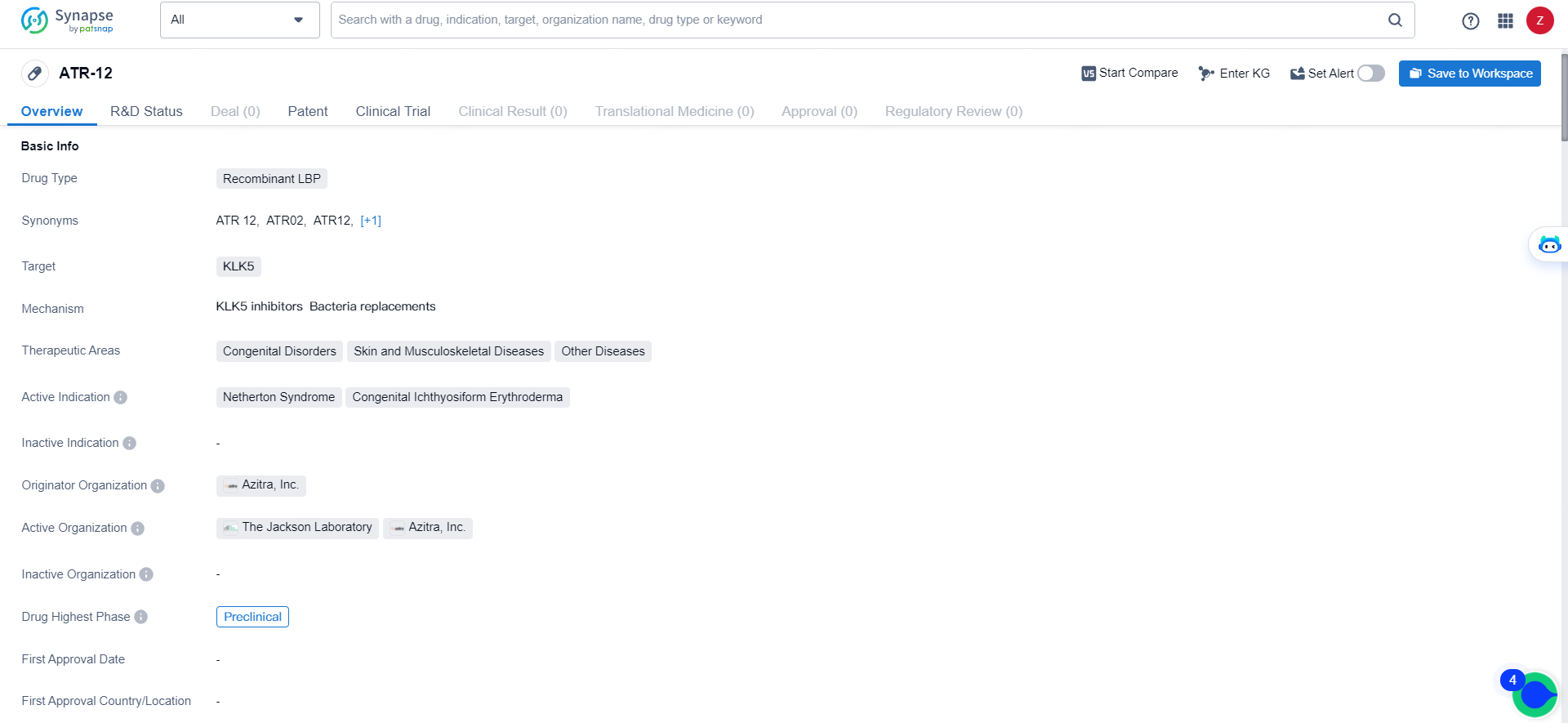
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
The findings presented in today's oral sessions highlight the preclinical advancements of ATR-12 and the clinical research framework for a Phase 1b study targeting Netherton syndrome patients. Laboratory data illustrate that ATR-12 secretes LEKTI protein capable of nanomolar inhibition of kallikrein (KLK) 5, a protease critical in Netherton syndrome. Additionally, in human ex vivo Netherton syndrome models, the supernatant of ATR-12 decreases protease activity around 7 times compared to healthy skin levels. In the ex vivo human skin models, ATR-12 resulted in more substantial LEKTI presence in the skin and deeper biodistribution after 24 hours compared to solely topically applied LEKTI.
Application of ATR-12 in human skin cell cultures led to a 92% reduction in IL-36γ, compared to extracts induced to overexpress IL-36γ. Furthermore, topical application of ATR-12 on in vitro human skin treated with erlotinib resulted in a 69% reduction of IL-36γ.
In trials with minipigs featuring abraded skin, topical application of ATR-12 led to LEKTI levels of 11.9 ng/cm² on the skin surface versus 2.6 ng/cm² in the control group at day 14. The use of ATR-12 was deemed safe and well-tolerated in GLP toxicology studies involving minipigs.
Another oral presentation titled “Staphylococcus epidermidis Strain Expressing LEKTI-D6 for Netherton Syndrome” outlined the clinical trial design for ATR-12 in Netherton syndrome patients. This Phase 1b trial is a multicenter, randomized, double-blind, vehicle-controlled study involving adults with Netherton syndrome.
"We are excited to share comprehensive preclinical data regarding ATR-12 and our clinical strategy for addressing Netherton syndrome, which underline the potential of genetically engineered skin commensals for protein delivery," stated Travis Whitfill, Azitra's co-founder and COO. "These findings validate the strong preclinical efficacy of ATR-12 in Netherton syndrome models and support the rationale for our Phase 1b clinical trial in Netherton syndrome patients."
ATR-12 (also known as ATR12-351) is an engineered S. epidermidis strain that produces a fragment of the human lympho-epithelial Kazal-type-related inhibitor (LEKTI) protein, absent in patients with Netherton syndrome, an often severe and chronic skin disease affecting roughly one to nine in every 100,000 people. ATR-12 is designed to deliver the deficient LEKTI protein when applied topically to patients. Azitra has an active IND for a Phase 1b clinical trial (NCT06137157) in adult patients. The company has established clinical sites and identified Netherton syndrome patients for participation in the upcoming 12-patient, Phase 1b trial, which aims to assess safety, tolerability, and efficacy. Preliminary safety data is expected to be announced before the end of the year.
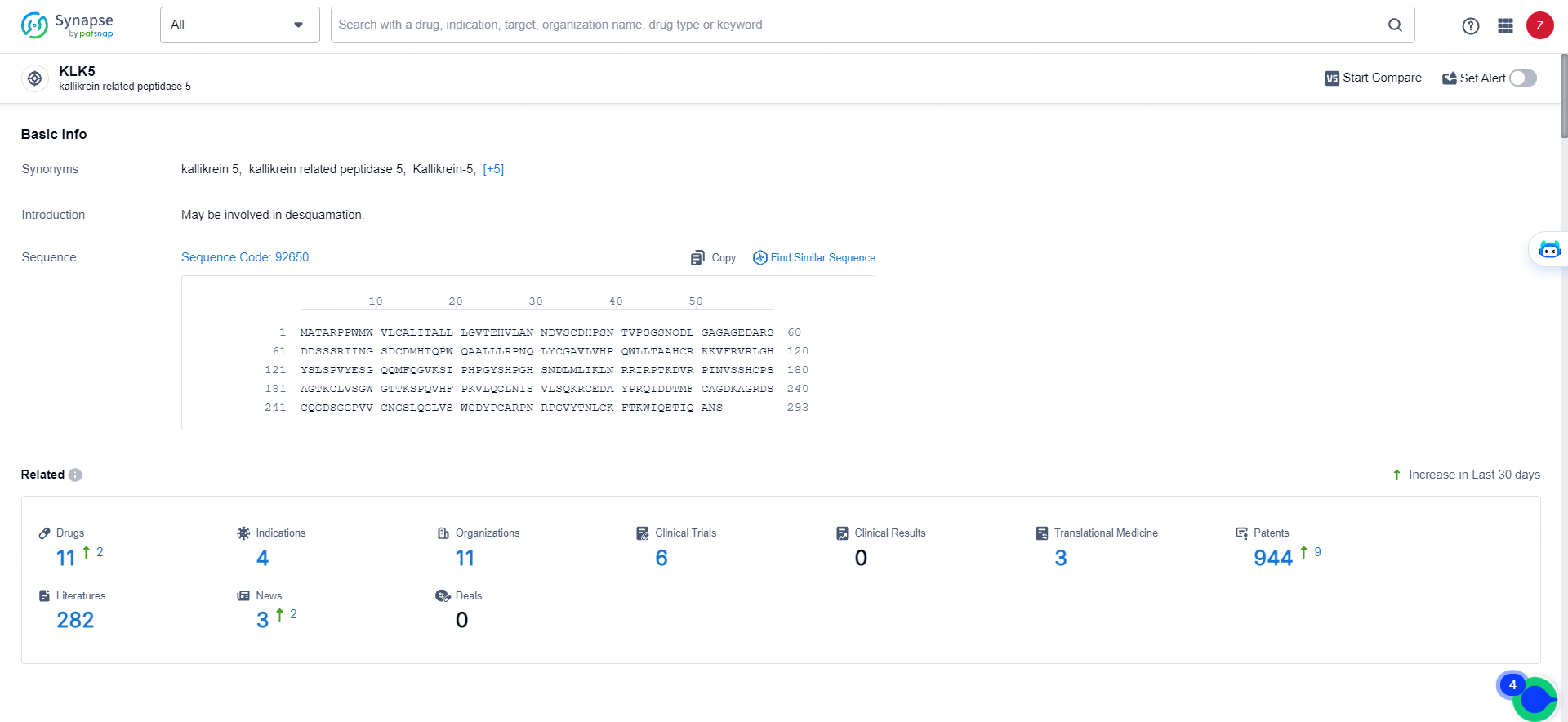
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of May 16, 2024, there are 11 investigational drugs for the KLK5 target, including 4 indications, 11 R&D institutions involved, with related clinical trials reaching 6, and as many as 944 patents.
ATR-12 is a recombinant LBP drug targeting KLK5, with a focus on treating congenital disorders, skin and musculoskeletal diseases, and other diseases. Its active indications include Netherton Syndrome and Congenital Ichthyosiform Erythroderma. Developed by Azitra, Inc., ATR-12 is currently in the preclinical phase of development, signifying its early-stage status in the drug development process.