Bayer Announces Promising NUBEQA® Phase III Results in Metastatic Hormone-Sensitive Prostate Cancer
The Phase III ARANOTE trial, researching the effects of NUBEQA (darolutamide) in combination with androgen deprivation therapy for those with metastatic hormone-sensitive prostate cancer, has achieved its primary goal of radiological progression-free survival. The combination of NUBEQA and ADT showed a statistically notable and clinically relevant improvement in rPFS when compared to placebo with ADT.
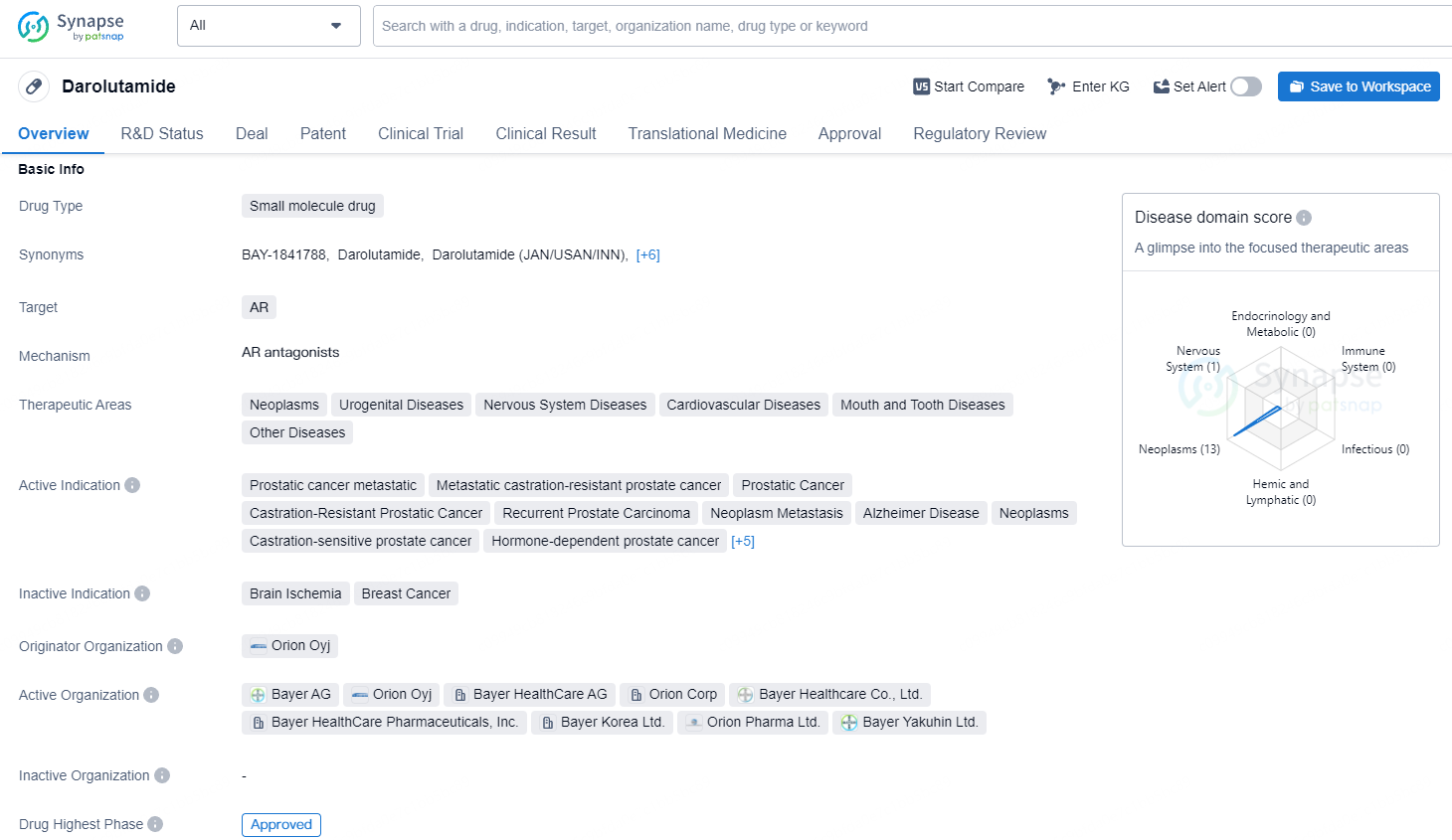
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
The findings were aligned with the established safety profile of NUBEQA, with no new safety concerns identified. Comprehensive results from this randomized, double-blind, placebo-controlled study are scheduled to be disclosed at an upcoming scientific meeting.
In the U.S., NUBEQA is authorized for treating adult patients with mHSPC in conjunction with docetaxel, as well as for those with non-metastatic castration-resistant prostate cancer.
"We are thrilled to present the favorable outcomes from this Phase III study. Pending potential regulatory endorsement, healthcare providers will have the flexibility to customize NUBEQA therapy plans with or without docetaxel depending on the specific needs of each patient," stated Christian Rommel, Ph.D., Head of Research and Development at Bayer's Pharmaceuticals Division.
NUBEQA® (darolutamide) is an androgen receptor inhibitor characterized by a unique chemical composition that competitively prevents androgen binding, AR nuclear translocation, and AR-driven transcription.
In the U.S., NUBEQA is approved for use with docetaxel and ADT in treating adult patients with mHSPC, and for those with non-metastatic castration-resistant prostate cancer undergoing ADT.
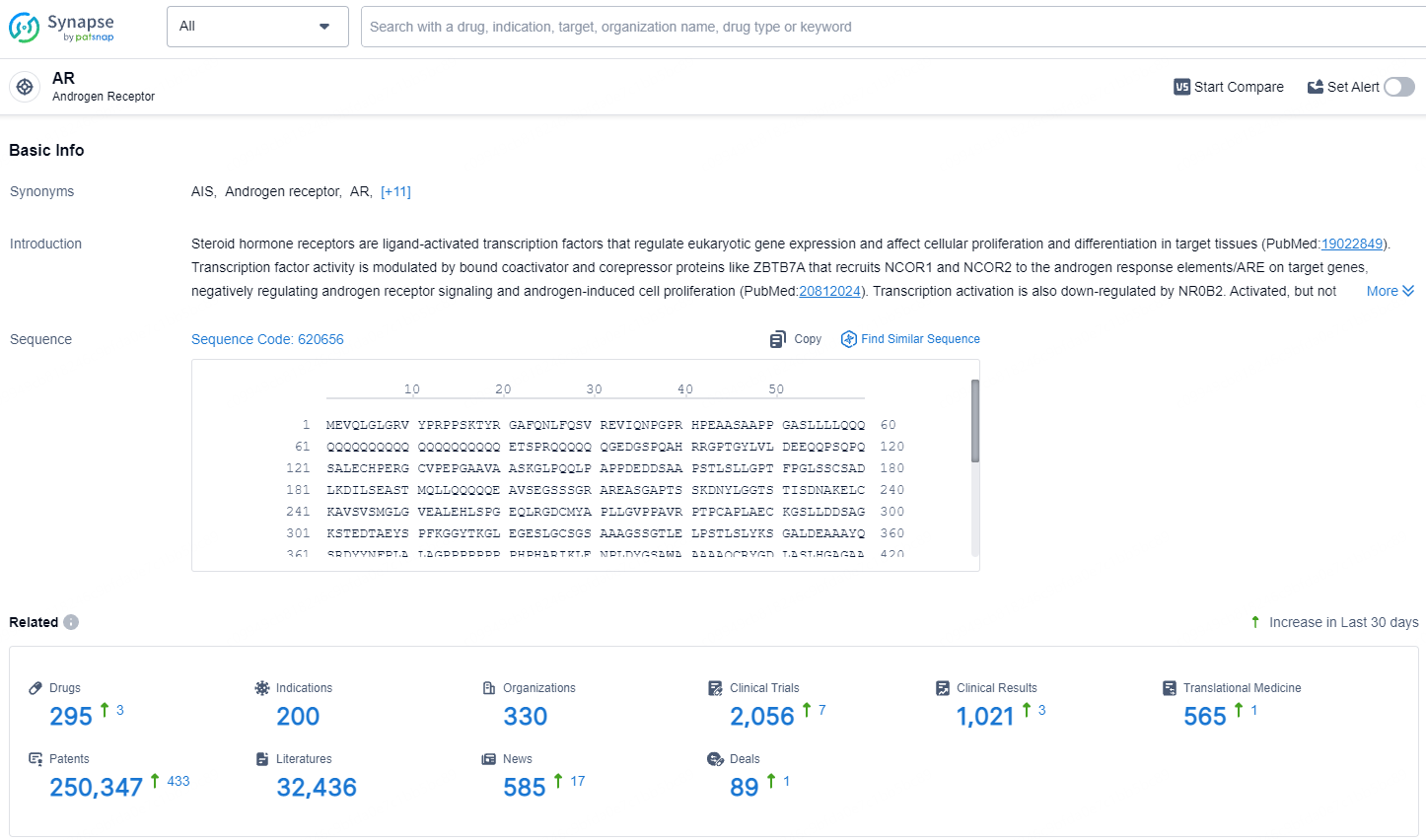
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of July 23, 2024, there are 295 investigational drugs for the AR target, including 200 indications, 330 R&D institutions involved, with related clinical trials reaching 2056, and as many as 250347 patents.
Darolutamide is a small molecule drug that targets the androgen receptor (AR) and has been approved for use in various therapeutic areas including neoplasms, urogenital diseases, nervous system diseases, cardiovascular diseases, mouth and tooth diseases, and other diseases. Darolutamide's approval and regulatory status place it as a significant player in the pharmaceutical industry and a noteworthy addition to the therapeutic options available for the treatment of a wide range of medical conditions.