Is Ganaxolone approved by the FDA?
Ganaxolone, marketed under the brand name Ztalmy, is FDA approved for the treatment of seizures associated with cyclin-dependent kinase-like 5 deficiency disorder (CDD) in individuals aged 2 years and older.
What is Ganaxolone?
Ganaxolone is classified as a gamma-aminobutyric acid (GABA) analog and is primarily used to manage seizures linked with CDD, a rare genetic disorder. It may also be prescribed for other purposes not specified in the medication guide.
Ganaxolone Side Effects:
Like all medications, ganaxolone can cause side effects. Common side effects may include drowsiness, fever, excessive saliva, or seasonal allergies. Serious side effects requiring immediate medical attention include allergic reactions and significant changes in mood or behavior, such as worsening depression or suicidal thoughts.
Warnings and Precautions:
Before starting ganaxolone, inform your healthcare provider about any history of liver disease, depression, suicidal thoughts, or alcohol/drug addiction. It's crucial to adhere strictly to prescribed dosages and to report any adverse effects promptly.
How to Take Ganaxolone:
Ganaxolone is administered orally as a suspension and should be taken with food to minimize gastrointestinal side effects. Dosage typically starts low and increases gradually over specified periods as tolerated, following a structured titration schedule.
Storage and Handling:
Store ganaxolone tightly closed in its original container at room temperature, away from moisture and heat. Unused medication should be discarded after 30 days.
What Happens if You Miss a Dose or Overdose?
If a dose of ganaxolone is missed, take it as soon as possible unless it is almost time for the next scheduled dose. In case of overdose, seek immediate medical attention or contact the Poison Help line.
Drug Interactions:
Discuss all medications, including over-the-counter drugs and herbal supplements, with your doctor before taking ganaxolone to avoid potential interactions that could affect its efficacy or increase the risk of side effects.
Conclusion:
Ganaxolone, available under the brand name Ztalmy, is an FDA-approved treatment for seizures associated with CDD in patients aged 2 years and older. It is essential to follow prescribed dosing guidelines, monitor for side effects, and report any concerns promptly to your healthcare provider.
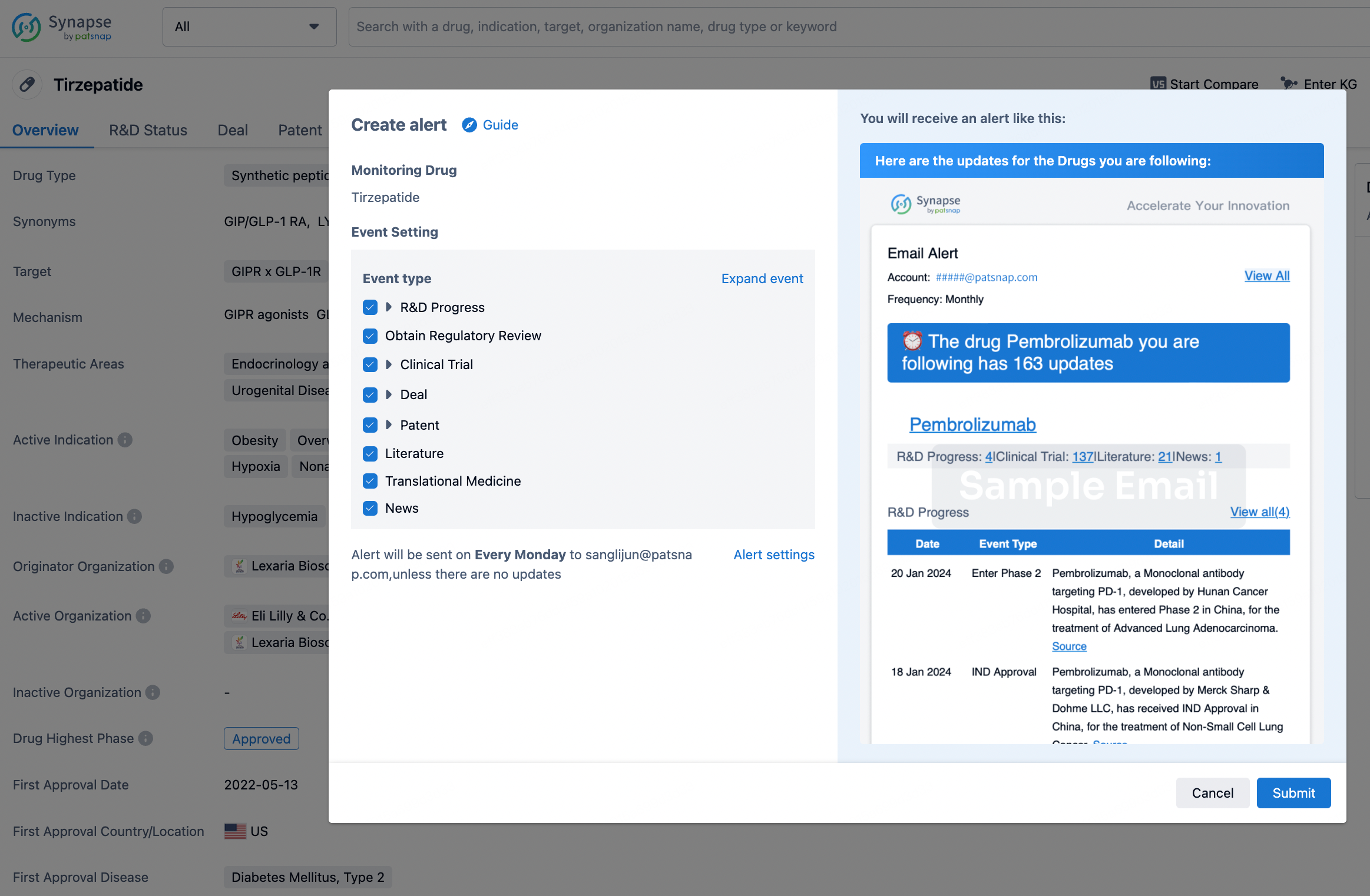
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!