Bayer has high expectations for Kerendia in treating heart failure
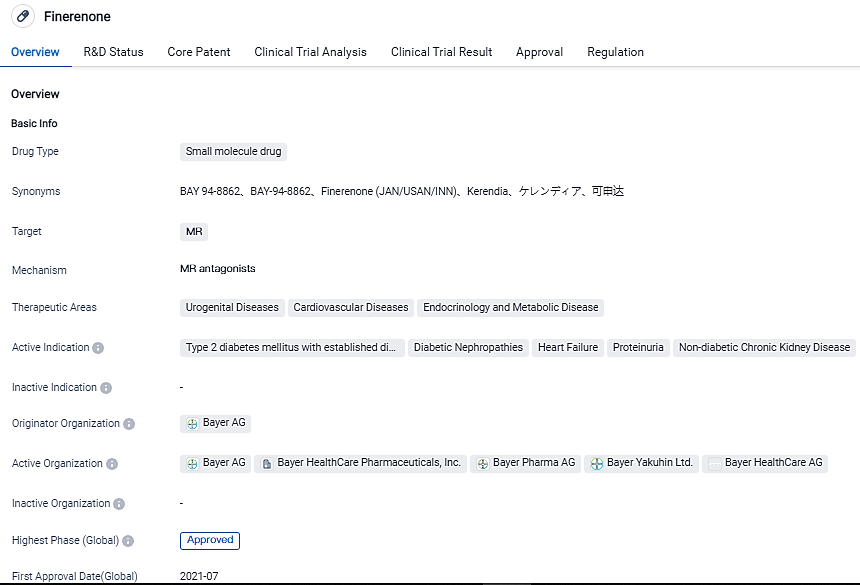
Bayer’sKerendia (finerenone) had gained approval for usage in managing chronic kidney diseases affiliated with type 2 diabetes two summers ago Nevertheless, this product did not emerge as the specified monetary blockbuster.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
During the second quarter, Kerendia registered sales of 67 million euros For Bayer to attach its goal of augmented sales at the peak of 3 bill euros, the extended usage of the non steroidal mineralogical receiver antagonist has to be allowed
On their journey towards this objective, Bayer is strategically increasing their efforts in the field of heart failure On Thursday, Bayer received its indications to launch three additional phases 3 trials for the indication, as from the one notionally in motion
The upbringing studies will apply Bayer's bug in approximately 9000 newly diagnosed heart failure patients with variable injection fractions - either reduced, slightly reduced or preserved.
The REDEFINE-HF research aims to explore the utilization of finerenone as a single treatment approach in an estimated 5,200 subjects with an ejection fraction exceeding 40%. The FINALITY-HF experiment is set to evaluate the effectiveness of finerenone as a sole therapy in approximately 2,600 subjects with an ejection fraction below 40%.
The transparency-based CONFIRMATION-HF research intends to analyze finerenone, paired with an SGLT2 inhibitor, against the standard healthcare approach in about 1,500 individuals who have been either admitted to hospitals or freshly released, suffering from HF, irrespective of their left ventricle ejection fraction levels.
These ongoing studies supplement the active FINEARTS-HF investigation, currently analyzing the effectiveness of finerenone compared to a placebo supplemented with a conventional healthcare regimen in 6,000 participants with marginally reduced or sustained ejection fraction levels.
"Our objective is to acquire a holistic understanding of finerenone's potential in treating heart failure by investigating its efficacy and safety in a wide range of patients and clinical environments," stated Bayer's Chief Medical Officer, Michael Devoy, in an announcement.
Two years prior, findings from the FINEARTS-HF research implied that Kerendia provided cardiac and renal advantages in heart failure patients who were concurrently taking an SGLT2 inhibitor. Beyond its evident potential in treating individuals with heart failure, Kerendia has demonstrated cardiovascular advantages within its approved uses.
In the previous year, Bayer introduced a pre-determined collective analysis of two trials involving patients with Chronic Kidney Disease (CKD) and type 2 diabetes, suggesting that Kerendia reduced the chance of sudden cardiac death by 25% compared to a placebo, and the likelihood of any cardiovascular event by 18%.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
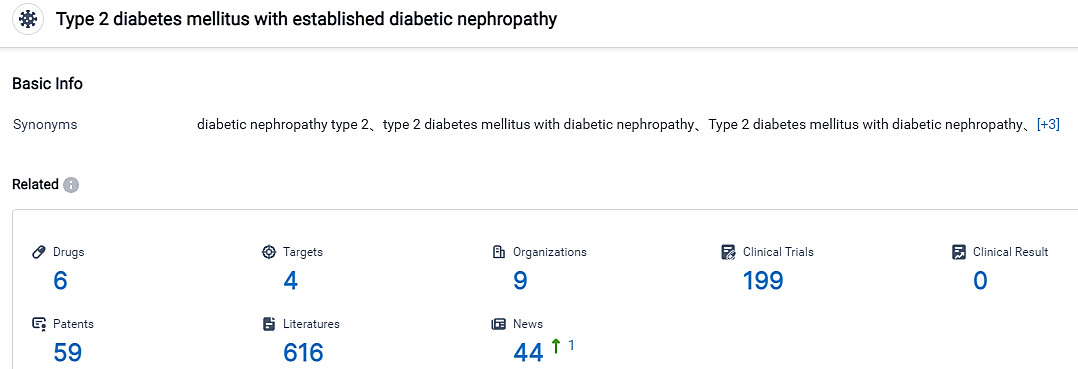
According to the data provided by the Synapse Database, As of September 5, 2023, there are 6 investigational drugs for the Type 2 diabetes mellitus with established diabetic nephropathy, including 4 targets, 9 R&D institutions involved, with related clinical trials reaching 199, and as many as 59 patents. Therapies for enduring heart malfunction are majorly characterized by extensive and routine use of well-known classes of drugs - these include angiotensin-converting enzyme inhibitors, along with beta-blockers.
Multidrug therapy comprising these antiquated drug classes is prevalent due to multifarious operational and structural complications which form the basis of heart failure syndrome. But in the preceding years, an array of advanced treatments has been developed specifically for chronic heart failure. These treatments have shown supplementary benefits in relation to cardiovascular results and thus, are deemed imperative in medical practice guidelines.
These treatments include Novartis’s Entresto (sacubitril/valsartan), Verquvo (vericiguat) developed by Merck and Bayer, and sodium-glucose cotransporter-2 (SGLT-2) inhibitors, Like Farxiga (dapagliflozin) by AstraZeneca and Jardiance (empagliflozin) by Eli Lilly and Boehringer Ingelheim. Patients are primarily categorized per ejection fraction into either diminished or retained populations. At present, therapies approved for those with reduced ejection fraction are more, yet the urgent need for therapies for patients with preserved ejection fraction is propelling novel research and is causing the approval of new drugs and label extensions.