Exelixis Unveils Positive Phase 3 Trial Results of Cabozantinib for Advanced Neuroendocrine Tumors
Exelixis, Inc. conveyed that the Alliance for Clinical Trials in Oncology's autonomous Data and Safety Monitoring Board unanimously agreed to bring the phase 3 CABINET benchmark trial to a halt and reveal the results sooner than initially planned because of highly significant improvements seen at a mid-stage review.
The CABINET trial is assessing the effects of cabozantinib (CABOMETYX©) versus a placebo for patients suffering from either advanced pancreatic neuroendocrine tumors or advanced extra-pancreatic neuroendocrine tumors, who have shown progression subsequent to previous systemic treatment. Cabozantinib was notable in extending the period without any progression of the disease or death in all trial groups.
The National Cancer Institute is sponsoring CABINET, which is directed by The Alliance for Clinical Trials in Oncology. Comprehensive results will be shared at a future medical conference and deliberated with the U.S. Food and Drug Administration.
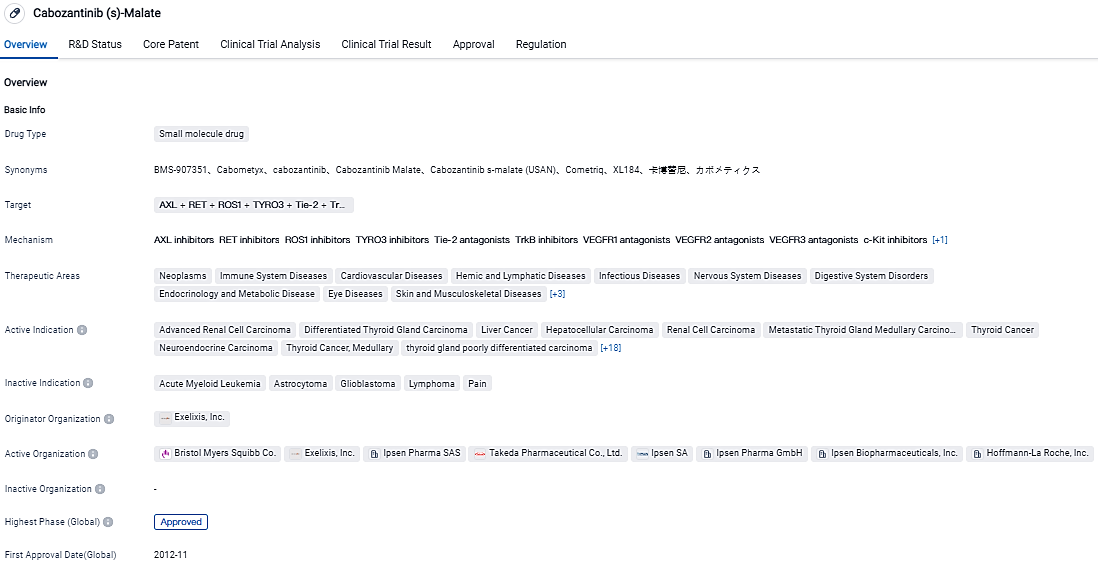
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
"With no established treatment protocol for patients with advanced pancreatic or extra-pancreatic neuroendocrine growths that have progressed following initial treatment, we are encouraged to observe that cabozantinib has been able to enhance outcomes for two more categories of patients struggling with advanced, hard-to-manage cancers," remarked Will Berg, M.D., Senior Vice President of Medical Affairs at Exelixis. "We appreciate the endorsement from the Data and Safety Monitoring Board to prematurely unmask the CABINET study on account of a major enhancement in efficacy and eagerly anticipate debating these discoveries with the U.S. Food and Drug Administration."
The safety norms of cabozantinib seen in the trial conformed to its recognized safety norms, and no new safety indicators were discovered.
"Neuroendocrine tumor patients who exhibit disease progression face constrained treatment alternatives. Currently, the trajectory of treatment following prior therapies is ambiguous, emphasizing the necessity for more options for this increasingly prevalent disease," expressed Jennifer Chan, M.D., M.P.H., who is leading the CABINET trial and also serves as the Clinical Director of the Gastrointestinal Cancer Center and chief of the Program in Carcinoid and Neuroendocrine Tumors at Dana-Farber Cancer Institute.
"The encouraging results from the CABINET trial, where cabozantinib provided an advantageous efficacy for patients with pancreatic and extra-pancreatic neuroendocrine growths, are a good sign and indicate the potential for cabozantinib to tackle key unsatisfied demands in this field."
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of September 4, 2023, there are 1 investigational drugs for the cabozantinib’s target, including 33 applicable indications, 8 R&D institutions involved, with related clinical trials reaching 272, and as many as 124 patents.
In the United States, CABOMETYX tablets have received approval for use in treating patients with an advanced stage of renal cell carcinoma, as a first-line treatment for advanced RCC in combination with nivolumab, and for both adults and children aged 12 and above with locally advanced or metastatic differentiated thyroid cancer that has worsened after prior VEGFR-targeted treatment and who are not suitable or have become resistant to radioactive iodine therapy.
Additionally, they have been approved for treating patients with hepatocellular carcinoma who were earlier treated with sorafenib. The approval for CABOMETYX tablets isn’t exclusive to the U.S., as they have received regulatory green light in the European Union and other additional worldwide locations.
Ipsen Pharma SAS was given exclusive rights by Exelixis in 2016 for the commercialization and further clinical development of cabozantinib outside the U.S. and Japan. The following year, Takeda was granted exclusive rights by Exelixis to commercialize and continue the clinical development of cabozantinib for any potential future applications only in Japan. The right to develop and sell cabozantinib within the U.S. still remains exclusively within Exelixis’s purview.