Beacon Therapeutics Announces Positive 24-Month Phase 2 SKYLINE Study Results for AGTC-501 in X-Linked Retinitis Pigmentosa
Beacon Therapeutics Holdings Limited, a prominent company specializing in gene therapy for eye conditions, aims to preserve and restore vision in individuals affected by vision-threatening retinal diseases. The Company recently shared interim safety and efficacy findings from the 24-month Phase 2 SKYLINE study focusing on XLRP. These results were presented at the American Academy of Ophthalmology's Annual Meeting in Chicago, Illinois.
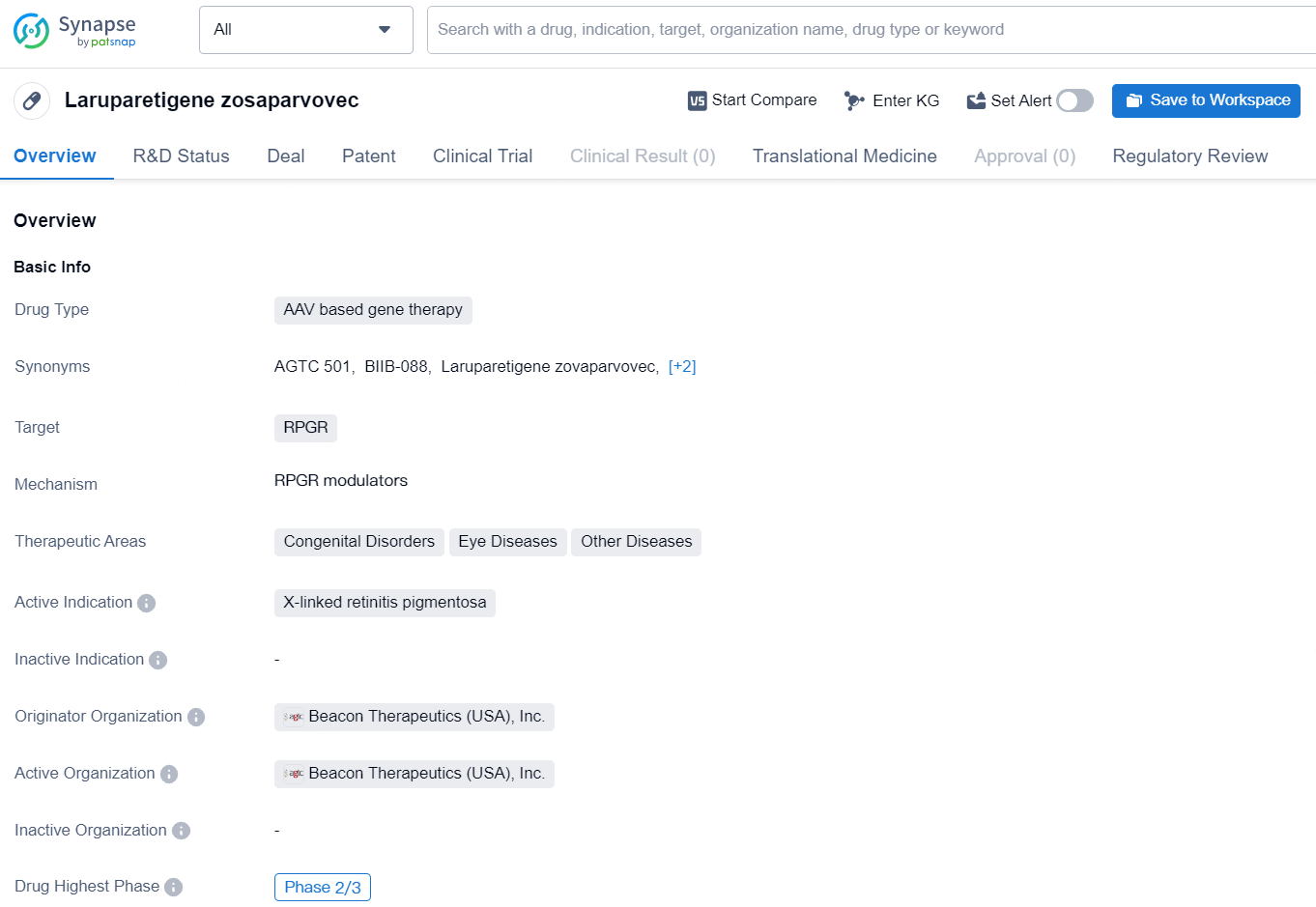
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The data from 24 months indicated a response rate of 57% (4 out of 7) in study eyes that received a high dose (6.8 E+11 vg/eye) of AGTC-501. This response is characterized by an improvement of at least 7 dB in retinal sensitivity as measured by microperimetry in a minimum of 5 locations. The patients in this high-dose group also exhibited a significant average enhancement in retinal sensitivity. Conversely, eyes receiving a lower dose (7.5 E+10 vg/eye) of AGTC-501 showed a 25% (1 out of 4) response rate. The trial reported no major safety concerns related to the AGTC-501 treatment, and any adverse events that emerged were mostly mild to moderate in nature.
XLRP is a severe and aggressive inherited retinal disease that frequently results in blindness by middle age, with no current treatment available. It primarily affects about 3.4 to 4.4 per 100,000 young males with RPGR mutations in the United States, Europe, and Australia. AGTC-501 is designed to express the full-length RPGR protein, addressing the comprehensive photoreceptor damage caused by XLRP, including both rod and cone degeneration. This makes it a promising potential treatment uniquely capable of improving the lives of XLRP patients.
Lance Baldo, MD, the CEO of Beacon Therapeutics, remarked, “Our 24-month Phase 2 SKYLINE data reaffirms AGTC-501’s strong safety profile and notable improvements in average retinal sensitivity. We remain committed to evaluating the prolonged safety and effectiveness of AGTC-501 and are hopeful due to the encouraging results observed so far in the SKYLINE trial.”
Beacon Therapeutics is currently enrolling participants in its pivotal Phase 2/3 VISTA trial as well as the open-label Phase 2 DAWN trial, advancing towards the development of a treatment for XLRP.
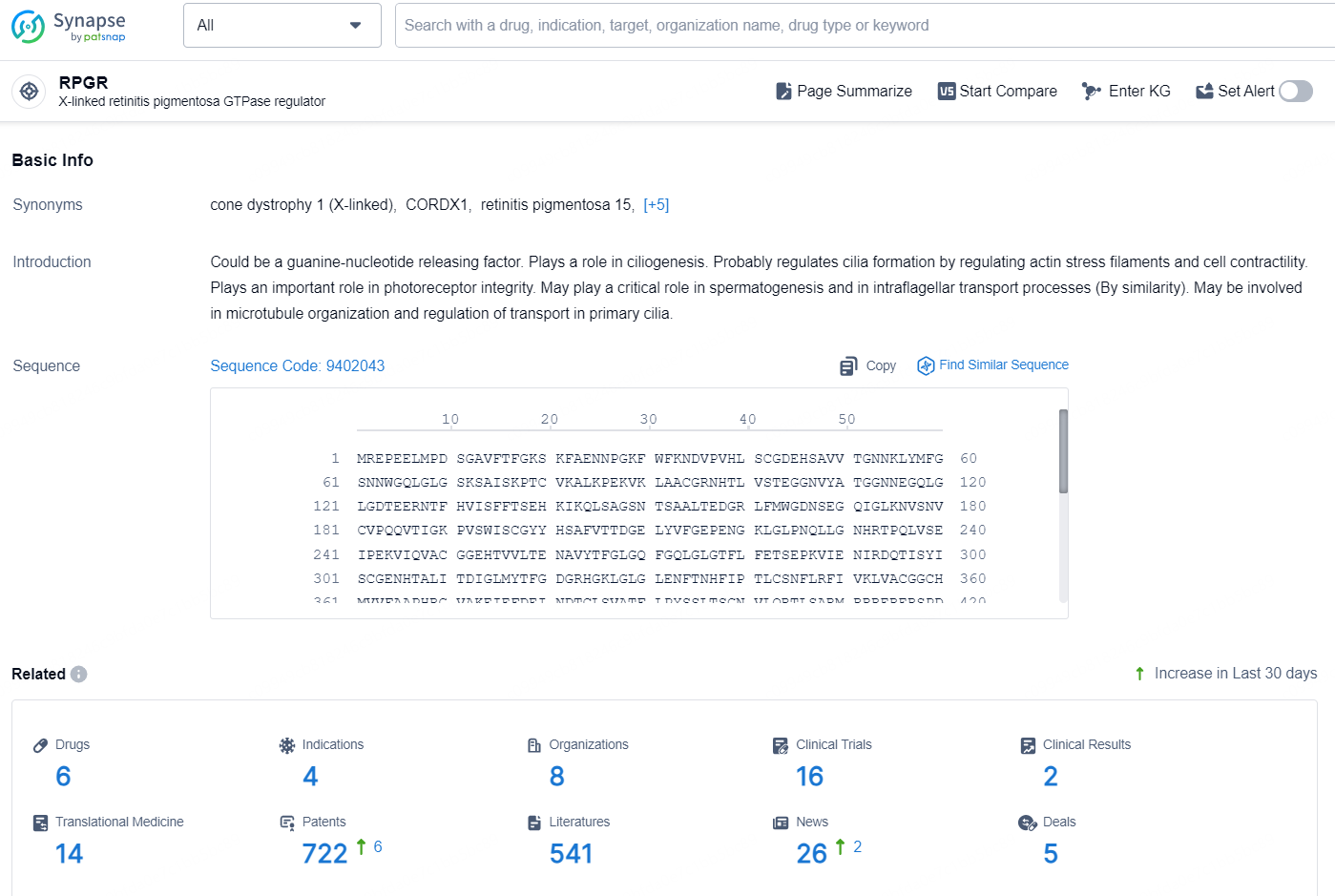
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of October 16, 2024, there are 18 investigational drugs for the RPGR targets, including 16 indications, 29 R&D institutions involved, with related clinical trials reaching 71, and as many as 4599 patents.
Laruparetigene zosaparvovec is an AAV (adeno-associated virus) based gene therapy developed by Beacon Therapeutics (USA), Inc. The drug is designed to target RPGR and is intended for the treatment of X-linked retinitis pigmentosa, a genetic disorder that causes progressive vision loss.