Biogen's SKYCLARYS® Wins EU Approval: A Breakthrough for Friedreich’s Ataxia
Biogen Inc. has revealed that the European Commission has granted approval for the use of SKYCLARYS® (omaveloxolone) as a therapy for adults and adolescents who are 16 years of age and above suffering from Friedreich’s ataxia. This regulatory clearance marks the initial authorization within the European Union for a medical treatment specifically targeting this uncommon, hereditary, and progressively worsening neurological disorder.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
During my time attending to patients, the severe effects of Friedreich's ataxia on individuals and their relatives have been evident to me,” remarked Sylvia Boesch, M.D., MSc, the leading research coordinator for the MOXIe investigation and director of the Rare Movement Disorders Center at Innsbruck's Neurological Department, Medical University of Innsbruck, Austria.
"Those who have Friedreich’s ataxia and participated in receiving SKYCLARYS within our studies observed substantial progress that genuinely enhanced their everyday experiences. With the recent endorsement, there is a wave of hope among stakeholders that SKYCLARYS will lead the way forward in handling Friedreich’s ataxia,” continued Sylvia Boesch.
"Biogen takes pride in incorporating SKYCLARYS into our suite of pharmaceutical offerings, presenting the inaugural therapy for individuals plagued by Friedreich’s ataxia across the European territory,” expressed Priya Singhal, M.D., M.P.H., executive of Biogen’s Development unit. “We extend our heartfelt gratitude to the entire Friedreich’s ataxia community for their pivotal role in propelling SKYCLARYS's journey to this pivotal authorization."
The European Commission's endorsement of SKYCLARYS stems from data attesting to its effectiveness and safety from the comparator-controlled second segment of the MOXIe trial. At the closure of the 48-week-long research, those administered SKYCLARYS showed a marked enhancement in their mFARS readings compared to those who received a placebo. Every domain of the mFARS evaluation, spanning swallowing capabilities, coordination of the upper and lower limbs, to maintaining an erect posture, indicated an advantage when utilizing SKYCLARYS as opposed to the placebo.
Further provisional findings originated from a post-study comparative review, demonstrating that individuals on SKYCLARYS during MOXIe presented lower mFARS outcomes after three years in contrast with a corresponding group tracked over natural disease progression. The most frequently recorded adverse reactions included elevations in liver enzymes, reductions in both weight and appetite, nausea, emesis, gastrointestinal upsets, headaches, fatigue, discomfort in the oropharyngeal region and back, muscular twitches, and instances of influenza.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
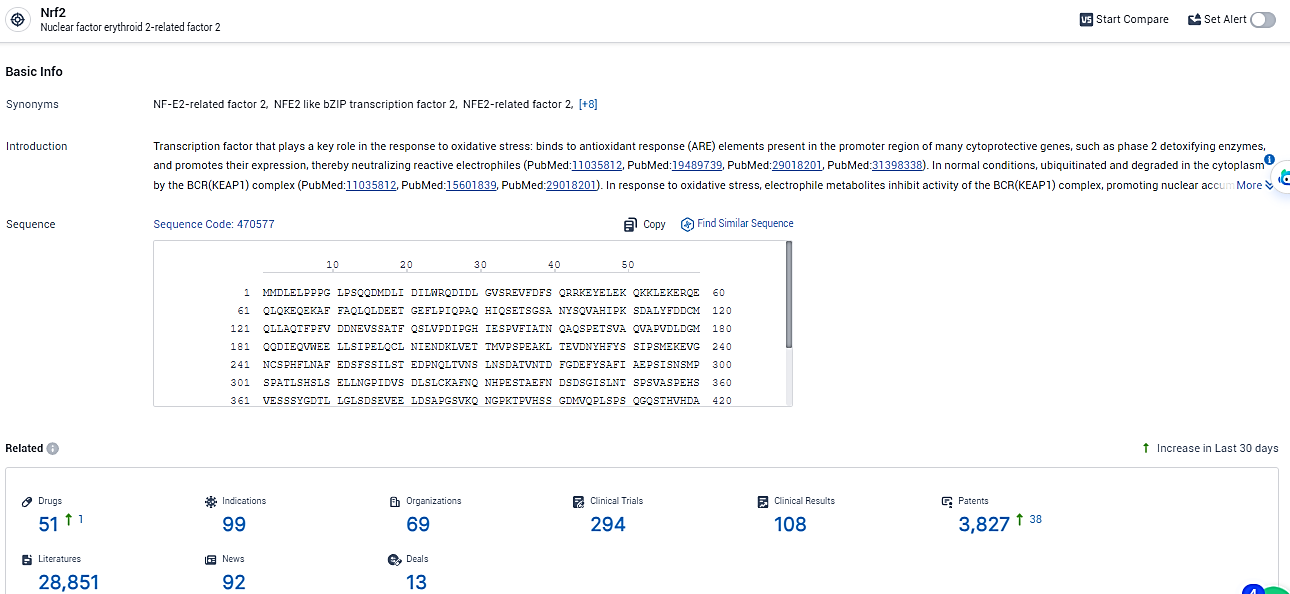
According to the data provided by the Synapse Database, As of February 19, 2024, there are 51 investigational drugs for the Nrf2 target, including 99 indications, 69 R&D institutions involved, with related clinical trials reaching 294, and as many as 3827 patents.
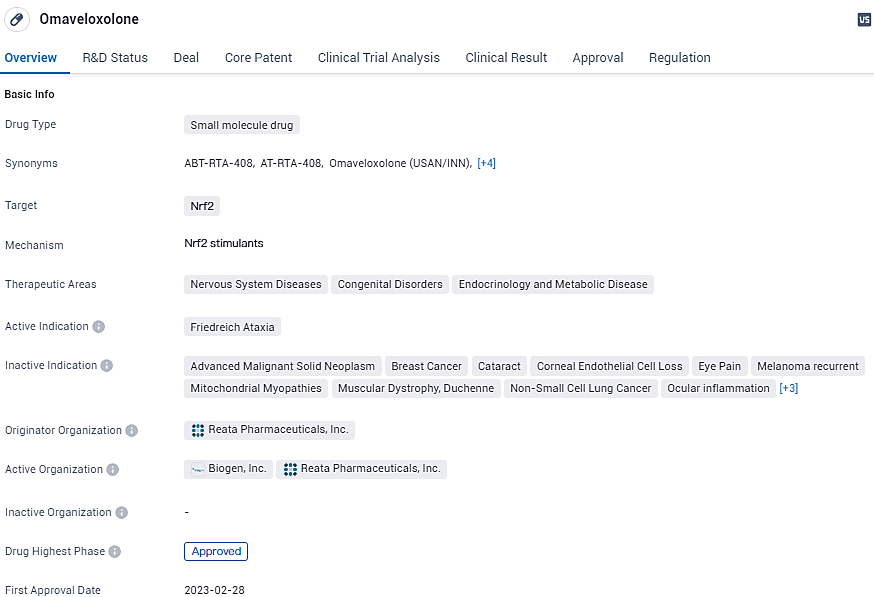
Omaveloxolone is a small molecule drug that targets Nrf2. It has received approval for the treatment of Friedreich Ataxia in the United States and holds regulatory designations such as Rare Pediatric Disease, Fast Track, and Orphan Drug. The drug shows promise in addressing various therapeutic areas, including Nervous System Diseases, Congenital Disorders, and Endocrinology and Metabolic Disease. Its approval marks a significant achievement for Reata Pharmaceuticals, Inc. and offers hope for patients suffering from rare Diseases.