Pimobendan: Detailed Review on its Transformative R&D Success, Mechanism of Action, and Drug Targets
Pimobendan's R&D Progress
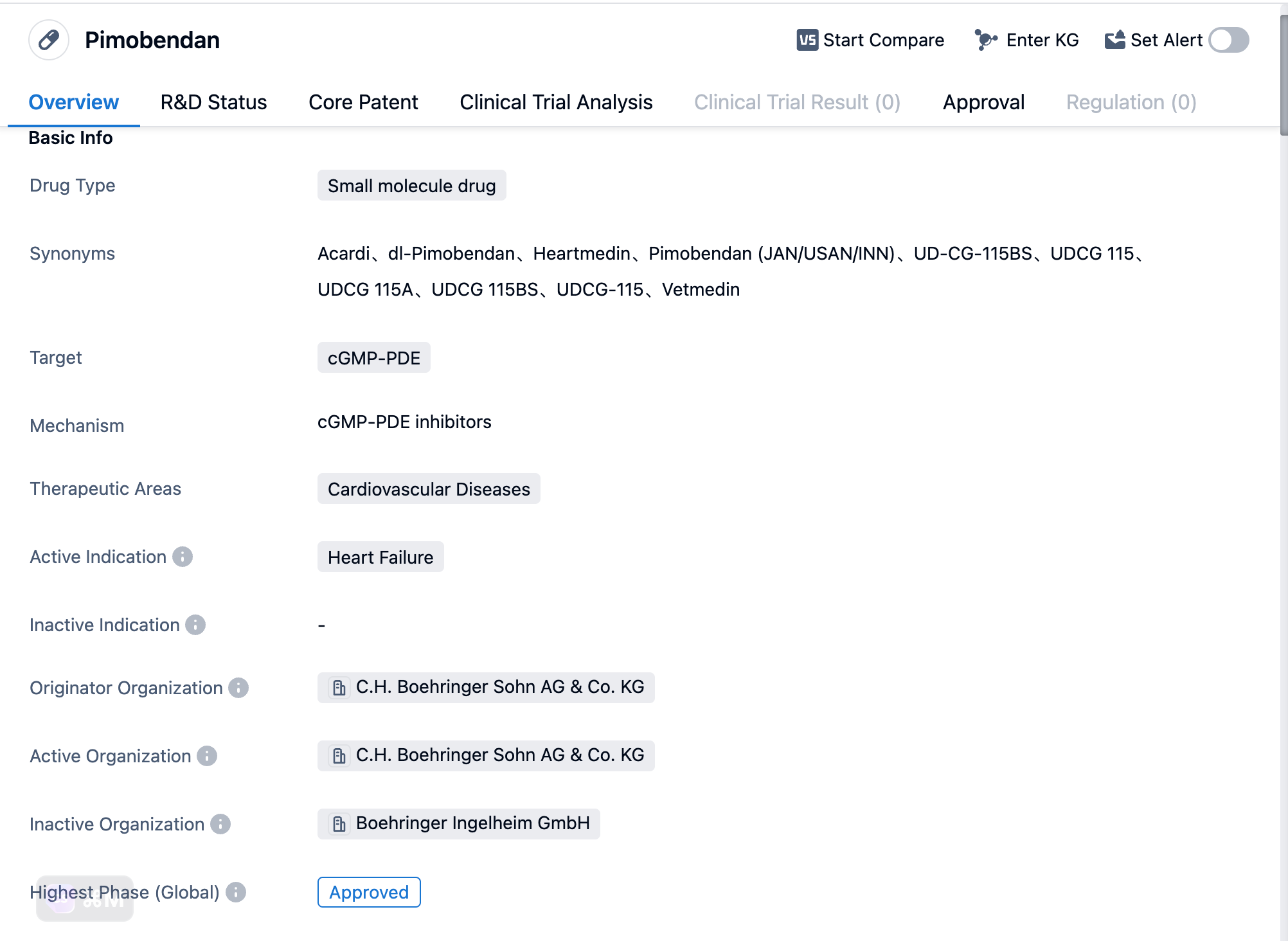
Pimobendan is a small molecule drug that falls under the therapeutic area of cardiovascular diseases. It specifically targets cGMP-PDE, which is an enzyme involved in the regulation of cyclic guanosine monophosphate (cGMP) levels in the body. By inhibiting this enzyme, Pimobendan is able to increase the levels of cGMP, leading to vasodilation and positive inotropic effects.
According to the data provided by the Patsnap Synapse-Global Drug Intelligence Database,the active indication for Pimobendan is heart failure, a condition characterized by the heart's inability to pump blood effectively. This drug has been approved for use in the treatment of heart failure, making it a valuable option for patients suffering from this condition.
Pimobendan was originally developed by C.H. Boehringer Sohn AG & Co. KG, a pharmaceutical company known for its expertise in the field of biomedicine. The first approval of Pimobendan took place in January 1994 in Japan. There is no more additional approvals in other countries or regions based on the information provided by Synapse.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Pimobendan: cGMP-PDE inhibitor
cGMP-PDE inhibitor is a type of drug that inhibits the enzyme phosphodiesterase (PDE) which specifically targets cyclic guanosine monophosphate (cGMP). This inhibitor prevents the breakdown of cGMP, leading to increased levels of cGMP in the body.
From a biomedical perspective, cGMP is an important signaling molecule involved in various physiological processes. It plays a crucial role in smooth muscle relaxation, vasodilation, and regulation of blood flow. By inhibiting the breakdown of cGMP, cGMP-PDE inhibitors promote the accumulation of cGMP, resulting in prolonged signaling and physiological effects.
In the context of biomedicine, cGMP-PDE inhibitors are commonly used to treat conditions such as erectile dysfunction, pulmonary arterial hypertension, and heart failure. By increasing cGMP levels, these inhibitors help relax smooth muscles, dilate blood vessels, and improve blood flow to specific areas of the body.
Drug Target R&D Development for Pimobendan
The drug target of Pimobendan is cGMP-PDE. According to PatSnap Synapse, as of 30 Aug 2023, there are a total of 44 cGMP-PDE drugs worldwide, from 57 organizations, covering 65 indications, and conducting 214 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.

According to the data provided by PatSnap Synapse, as of 30 Aug 2023, The drug types progressing most rapidly under the current target cGMP-PDE are Small molecule drugs and AAV-based gene therapy. Small molecule drugs have the highest number of approved drugs, with a total of 17. There is also one Small molecule drug in the Preclinical phase. Other drug types include Chemical drugs and Unknowns, which have limited representation.

Conclusion
In summary, Pimobendan is a small-molecule drug that targets cGMP-PDE and is used in the treatment of heart failure. It was developed by C.H. Boehringer Sohn AG & Co. KG and received its first approval in Japan in January 1994.