Bristol-Myers Squibb’s mavacamten has announced long-term follow-up data from two phase 3 trials for the treatment of hypertrophic cardiomyopathy
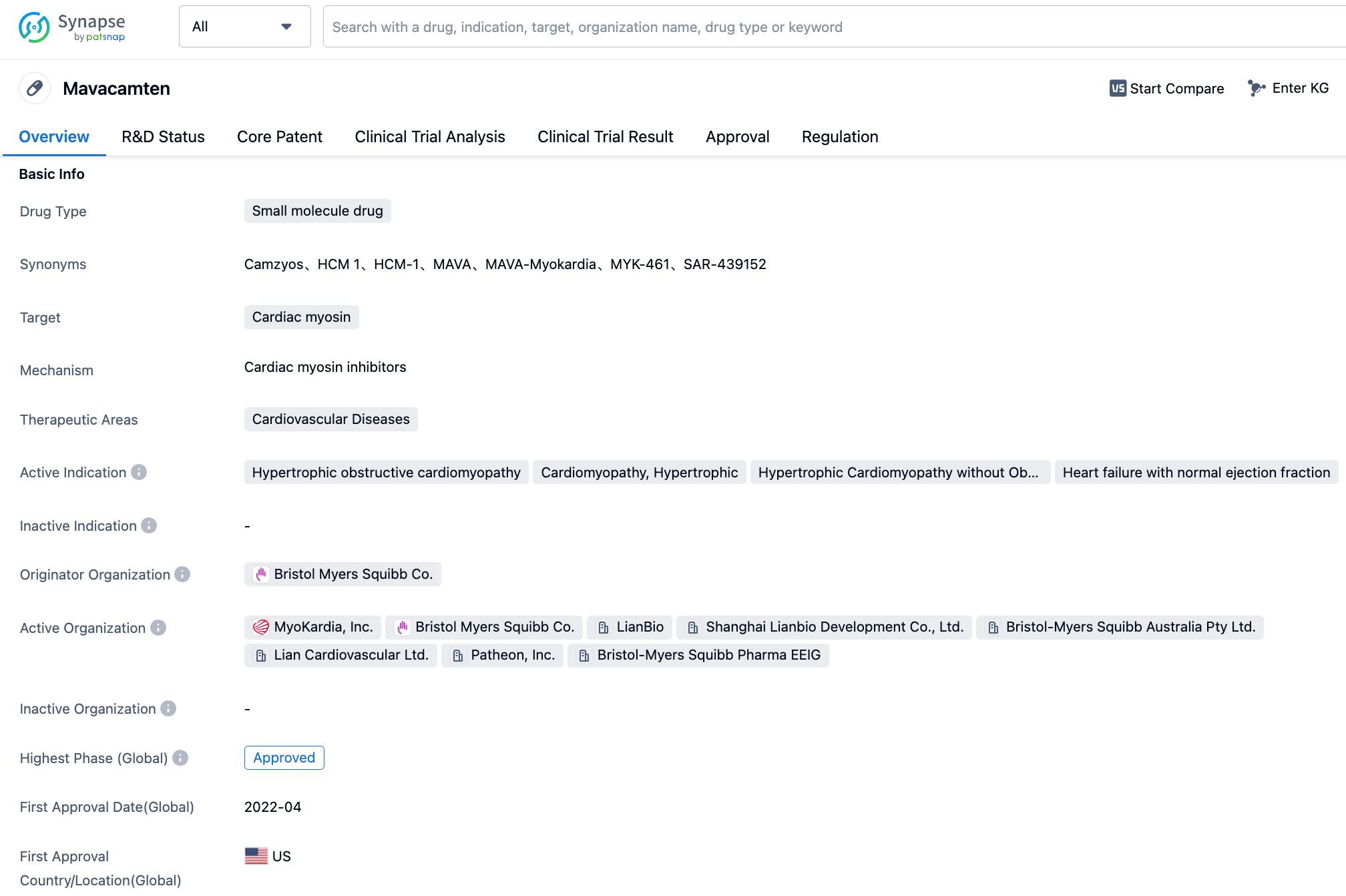
Recently, Bristol Myers Squibb has announced the latest long-term follow-up results of two Phase 3 studies that assessed the treatment efficacy of Camzyos (mavacamten) on symptomatic obstructive hypertrophic cardiomyopathy (oHCM) in adult patients.
Mavacamten is a selective Cardiac myosin conformer inhibitor developed by Bristol Myers Squibb. It reduces the ATPase activity of the cardiac myosin heavy chain, inhibits the excessive formation of myosin-actin cross-links, and induces the entire myosin to transition into a super relaxed state. Consequently, it prevents excessive myocardial contraction and improves diastolic compliance and energy metabolism. In April 2022, mavacamten received FDA approval for the treatment of symptomatic Class II-III obstructive HCM in adult patients to improve their cardiac function and symptoms. Currently, mavacamten has obtained marketing approval from regulatory authorities in Australia, Canada, Brazil, Switzerland, Macau, China, and Singapore.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The results of two phase 3 studies showed that with the extension of follow-up time, Camzyos continued to reduce the proportion of patients needing to undergo invasive septal reduction therapy (SRT) at 56 weeks. The cumulative analysis of up to 120 weeks showed persistent improvement in LVOT obstruction, symptoms, and NT-proBNP levels in symptomatic oHCM patients, with no new safety signals observed.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
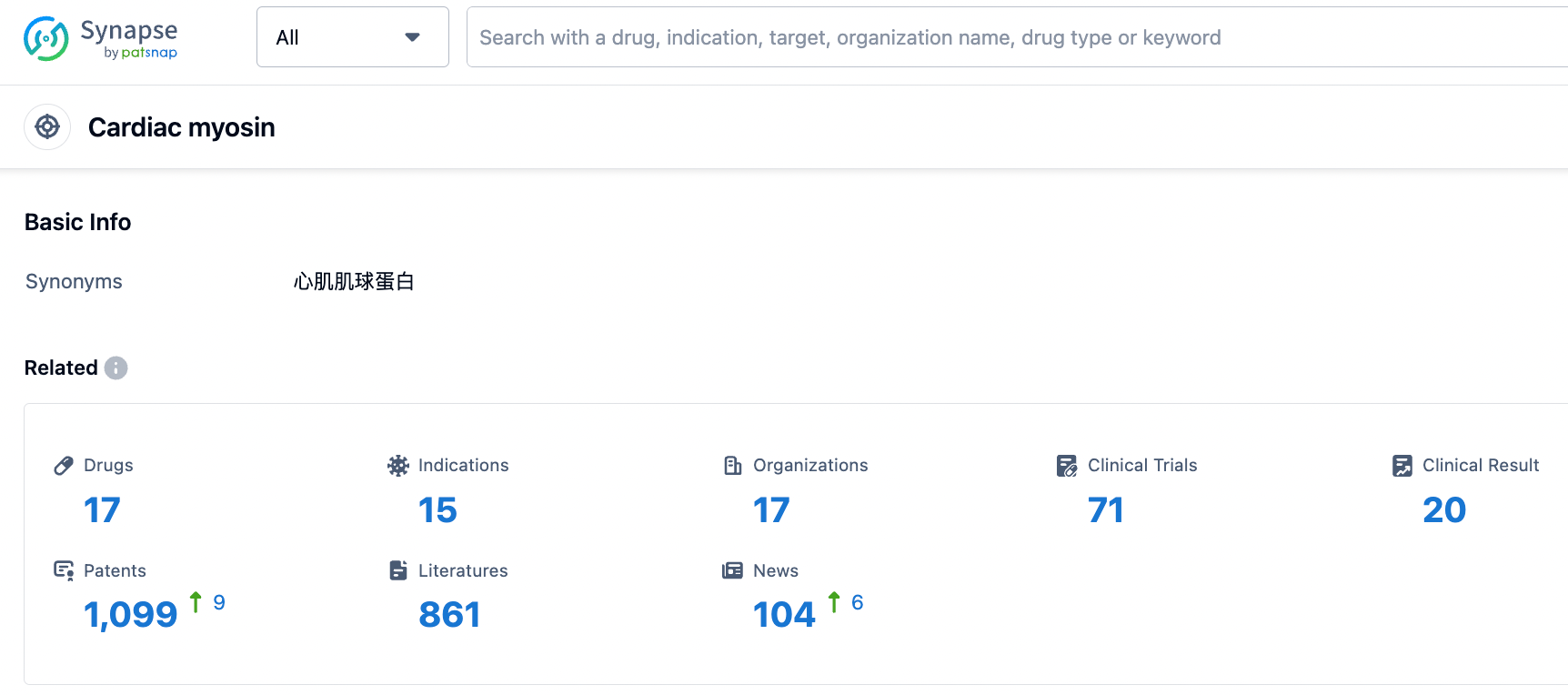
According to information disclosed from the Synapse database, as of September 1, 2023, there are a total of 17 drugs under research targeting Cardiac myosin, with 15 intended indications, 17 research institutions involved, 71 related clinical trials, and as many as 1100 patents.Hypertrophic cardiomyopathy (HCM) is a common monogenic hereditary disease in clinical practice, with an incidence rate of about 0.2%. It is hoped that mavacamten can be approved as soon as possible, bringing new treatment options for patients.