Breaking Through the Blood-Brain Barrier: P-Selectin-Targeted Nanocarriers Promote Active Crossing
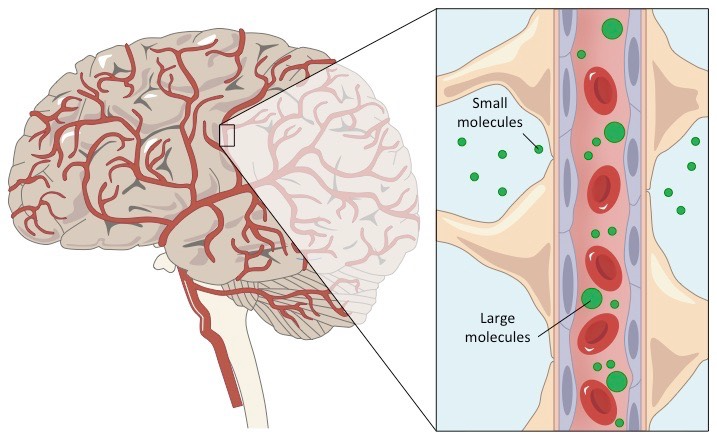
The blood-brain barrier (BBB) is a highly selective, semipermeable endothelial boundary that restricts the nonspecific entry of solutes from circulating blood into the extracellular fluid surrounding neurons in the central nervous system. The BBB's unique regulatory properties pose challenges for the targeted delivery of therapeutics to brain parenchyma. Tight junctions between adjacent brain endothelial cells prevent paracellular transport, hindering drugs from directly accessing lesioned brain tissue. While many lipophilic drugs can enter by passive diffusion, their penetration into diseased brain tissue is often poor, necessitating high doses that can lead to substantial systemic toxicities.
Nanocarrier to cross cellular barrier
Medulloblastoma patients in the Sonic hedgehog medulloblastoma subgroup (SHH-MB) face a daunting challenge as they endure poorer clinical outcomes. This is partly due to the stubborn blood-brain barrier (BBB), which impedes drug penetration into the brain at therapeutic concentrations. To make matters worse, targeted inhibition of the SHH effector Smoothened (SMO) using vismodegib can lead to severe toxicities and premature growth plate fusion.
A team of researchers from Memorial Sloan Kettering Cancer Center in New York decided to take on this challenge, investigating active transcellular transport mechanisms to enhance drug delivery across the BBB directly to tumor tissue.
They are certainly not alone in considering nanocarriers for help. Actually, a Synapse search indicates that major pharmaceutical companies are actively experimenting this technology.
The only previous clinical trial involving nanocarriers has failed and has been withdrawn since:
Good news is that another previous research has established P-selectin-targeted nanocarriers, based on the fucoidan polysaccharide for activated endothelium, which revealed that radiotherapy (RT) could increase endothelial P-selectin expression. This resulted in greater accumulation of nanocarriers at the tumor site. P-selectin aids in the transcellular transport of materials through caveolin-1 (Cav1)-mediated transcytosis across an intact BBB.
In a SHH-MB genetically engineered mouse (GEM) model with an intact BBB, the researchers discovered that P-selectin targeting led to active transcytosis in tumor endothelium. This allowed fucoidan-based nanocarriers to selectively deliver cargo to the tumor microenvironment. Fucoidan nanocarriers encapsulating the inhibitor vismodegib (FiVis) exhibited potent inhibitory effects at low drug doses, with significant antitumor efficacy and the ability to reduce on-target bone toxicities.
Moreover, this mechanism may also enhance drug delivery across an intact BBB to activated brain endothelium in other intracranial pathologies, offering hope for patients facing similar challenges.
Targeting P-selectin
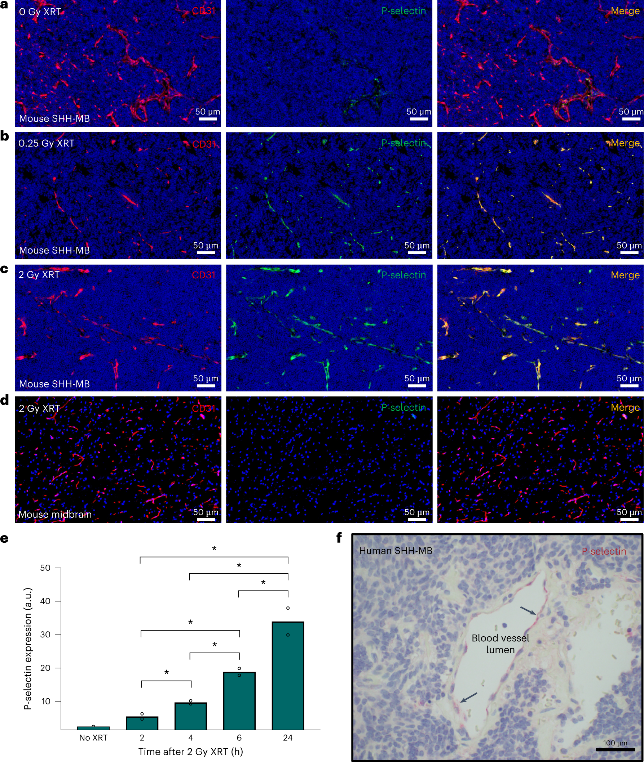
The researchers embarked on a journey to uncover the intricate workings of P-selectin expression in the SHH-MB tumor microenvironment and the effects of low-dose x-ray irradiation (XRT). Through rigorous experimentation, they discovered that whole-brain XRT increased P-selectin expression in the tumor region, while causing minimal effects in the adjacent normal cerebrum. To avoid RT-related toxicities, minimum required dose was used to enhance endothelial P-selectin expression, and a single 0.25 Gy XRT dose proved to elevate P-selectin, with significant increases observed about six hours post-XRT that persisted for at least 24 hours.
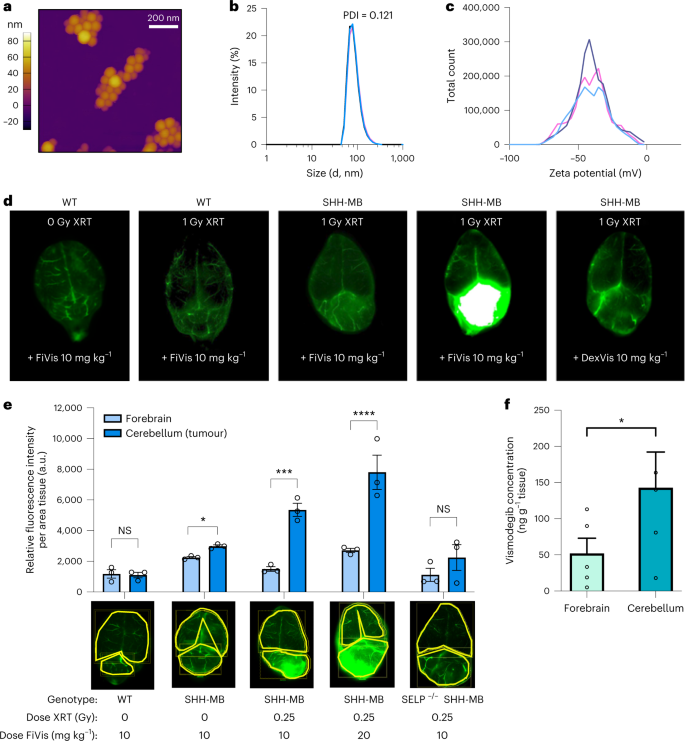
The nanocarriers, FiVis, were then crafted, by conjugating fucoidan polysaccharide and near-infrared dye (IRDye783), with an average diameter of 80±10 nm. The targeting and effects of radiation on FiVis delivery in SHH-MB tumors were evaluated. In unirradiated mice treated with FiVis, experiments showed slightly increased targeting to cerebellar tumors over the adjacent normal cerebrum. However, in mice pretreated with radiation (1Gy), significant FiVis accumulation was observed at the tumor site compared to wild-type (WT) mice without tumors following FiVis administration.
The researchers then delved into the transcellular transport mechanisms of FiVis across the BBB. After observing increased expression of Cav1 in tumor endothelium in mice treated with FiVis, they applied pharmacological inhibition of either caveolin- or clathrin-dependent endocytosis using methyl-β-cyclodextrin (CD; caveolar-dependent endocytosis inhibitor) or chlorpromazine (CPZ; clathrin-mediated endocytosis inhibitor) respectively, to bEnd.3 cells.
Results showed FiVis uptake significantly reduced in a dose-dependent manner after CD treatment, indicating that FiVis entry into mouse brain endothelial cells is mediated by caveolin-dependent endocytosis. Further studies were conducted to examine caveolin-mediated endocytosis using Cav1 knockout (KO) bEnd.3 cells. The results showed a significant decrease in FiVis uptake compared to WT cells.
Enhancing Drug Delivery
In order to uncover the secrets of FiVis delivery to SHH-MB tumors, the researhcers propagated SHH-MB tumors lacking Cav1 in mice. After low-dose XRT, FiVis nanocarrier accumulation reduced in the tumor region of these Cav1KO SHH-MB mice, despite their presence within the tumor vasculature. These findings illuminated the intricate mechanisms of FiVis delivery to SHH-MB tumors and offered hope for overcoming the daunting challenge posed by the BBB.
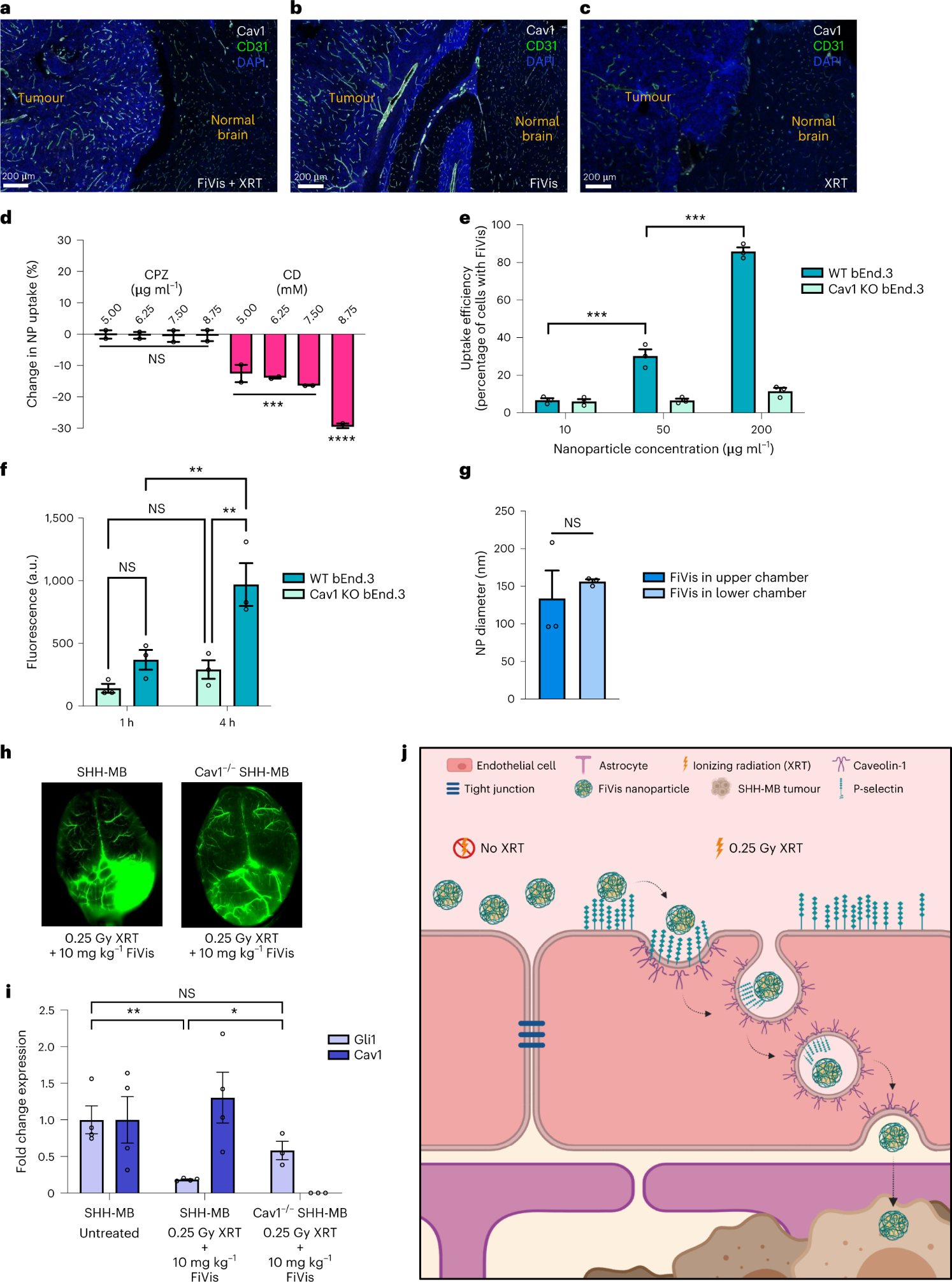
The therapeutic efficacy of FiVis in the SHH-MB GEM model was then assessed. Mice with established tumors were treated with FiVis or free vismodegib at an equivalent dose. The results were astounding - FiVis treatment significantly inhibited tumor growth compared to free vismodegib. Importantly, FiVis treatment was associated with an absence of premature growth plate fusion, a common adverse effect of vismodegib therapy, indicating reduced bone toxicity. Pre-treatment with XRT further enhanced FiVis delivery and therapeutic efficacy, with significant inhibition of tumor growth even at a lower drug dose. Survival analysis revealed that mice treated with FiVis at a lower dose survived significantly longer than those treated with free vismodegib.
To gain a better understanding of the mechanism of FiVis delivery, in vivo imaging was conducted. Fluorescence microscopy of brain sections from mice treated with FiVis revealed a greater accumulation of the nanocarrier in the tumor region compared to the adjacent normal cerebrum. This confirmed the preferential delivery of FiVis to the tumor site.
Fucoidan-based nanocarriers can actively transport therapeutic cargo across tumor endothelium, allowing for targeted delivery to the tumor microenvironment. This approach holds immense potential to revolutionize therapeutic outcomes for diseases with an intact BBB, such as SHH-MB, and could usher in a new era of improved therapies for a multitude of brain diseases.

Reference
Tylawsky, D.E., Kiguchi, H., Vaynshteyn, J. et al. P-selectin-targeted nanocarriers induce active crossing of the blood–brain barrier via caveolin-1-dependent transcytosis. Nature Materials 22, 391–399 (2023).