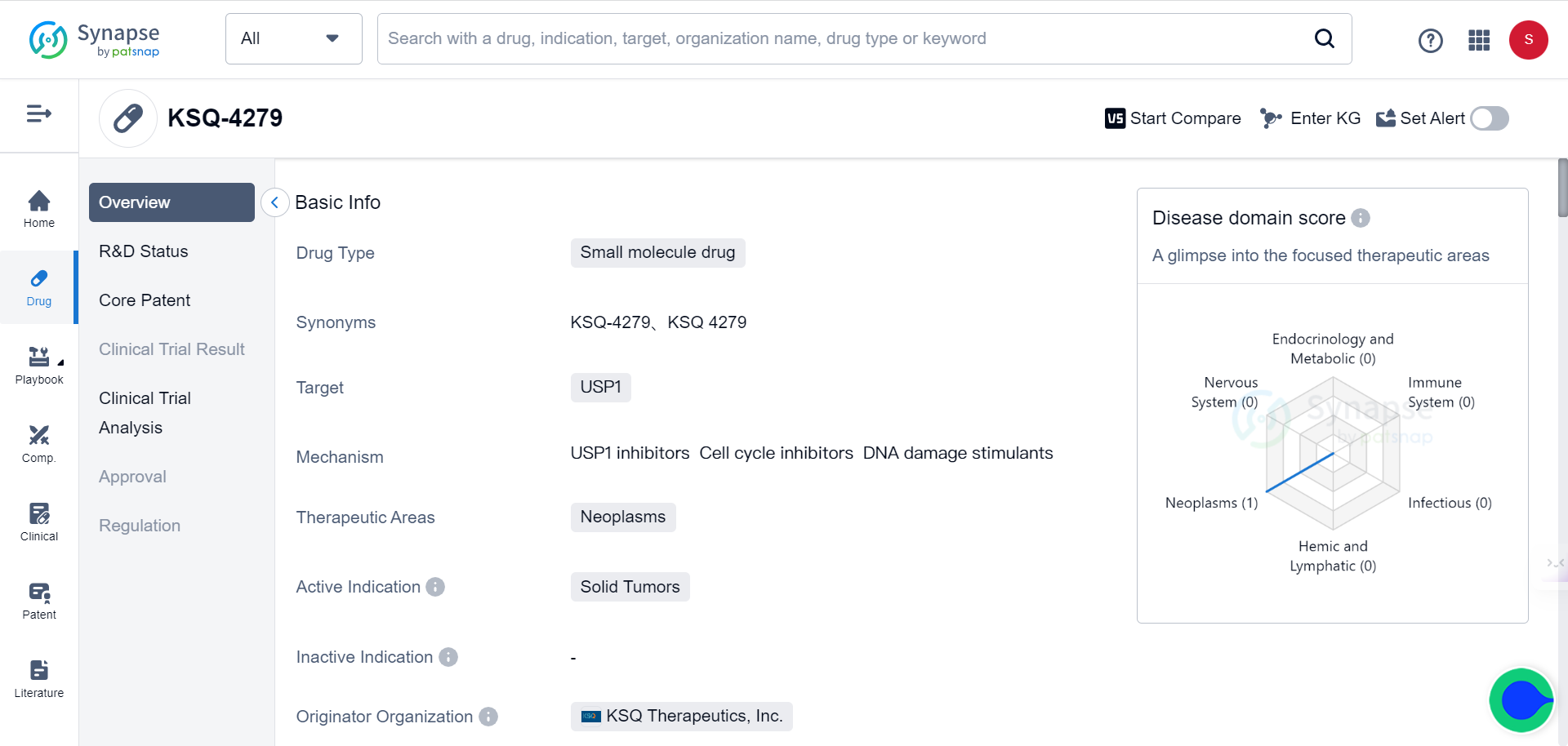
The novel class of USP1 inhibitors opens up new frontiers for the treatment of cancers and diabetes
KSQ Therapeutics has entered a worldwide licensing and collaboration agreement with Roche for the development and commercialization of KSQ-4279, a first-in-class, potent, and selective small molecule inhibitor of USP1. USP1 is a protein that regulates the DNA damage response (DDR) in a novel way distinct from other approaches, including PARP inhibitors. The molecule is currently in Phase 1 clinical trials for the treatment of solid tumors. Under the collaboration, Roche will assume development responsibilities for KSQ-4279, which has the potential to treat various cancers. Inhibiting USP1 activity with small molecules like this represents a pathway with potential to address unmet needs in oncology.
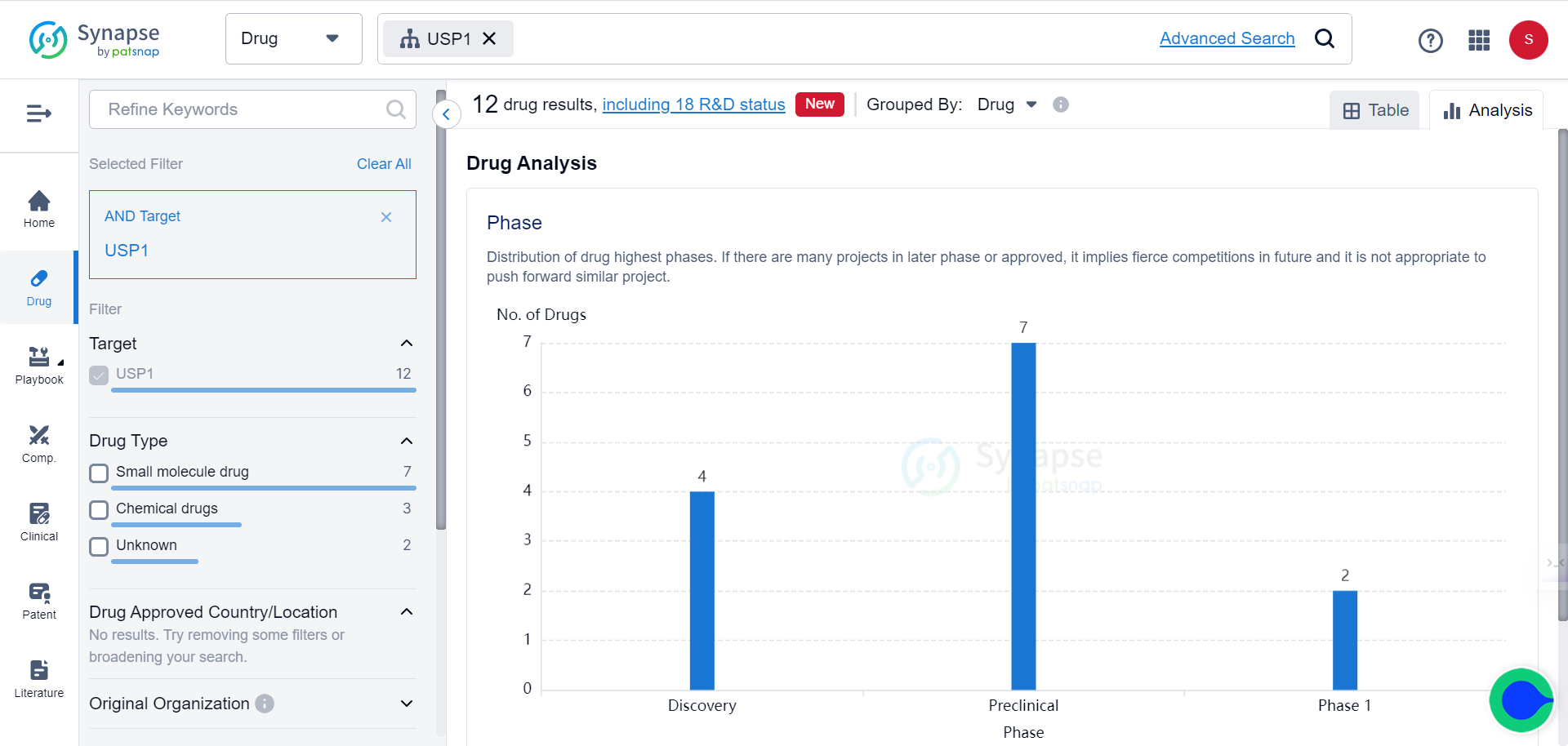
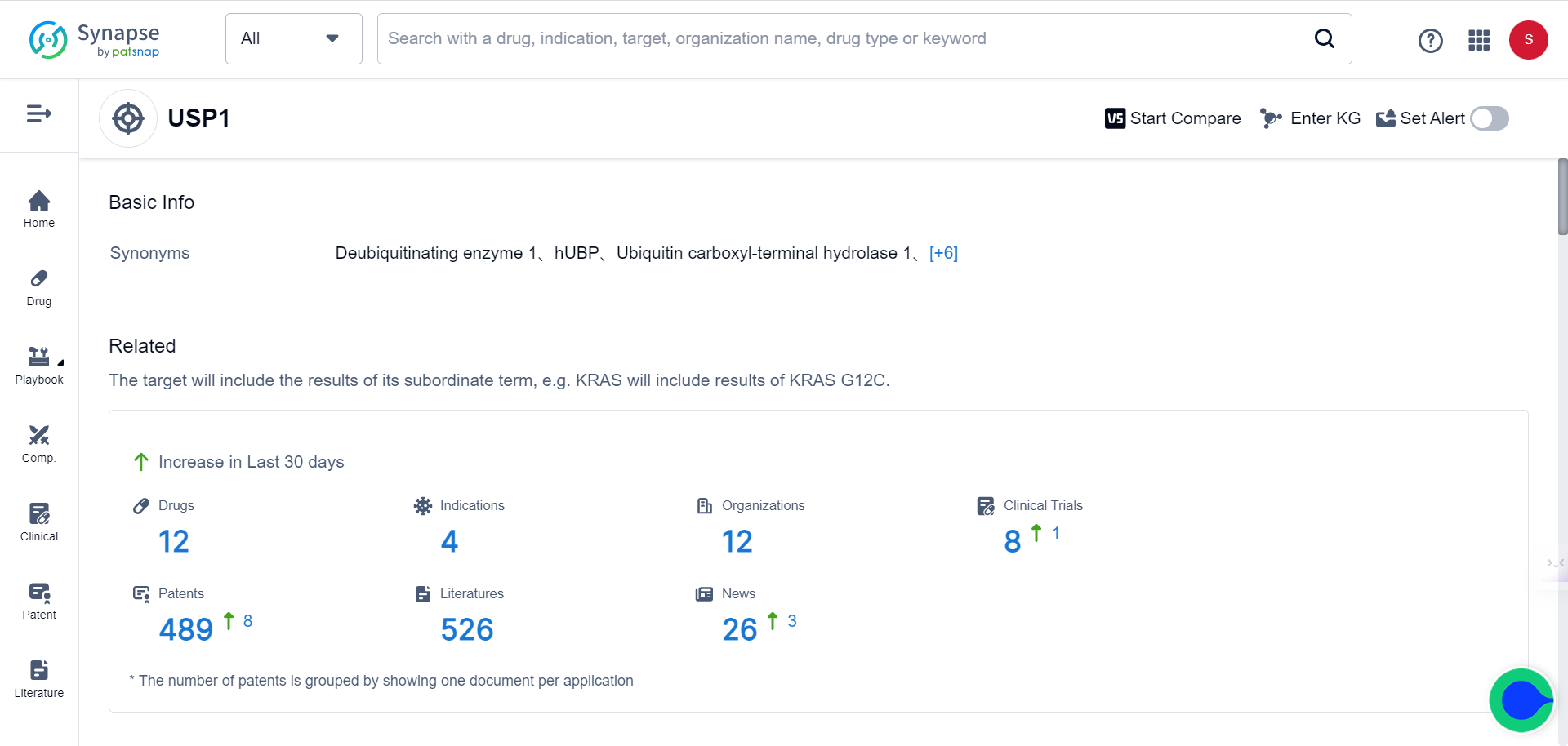
The Synapse database indicates that there are currently 12 drugs targeting USP1 under various stages of development.
To appreciate the potential of this novel target, note that 8 patents related to USP1 have been filed in just the past 30 days.
The mechanism of USP1 inhibition
Studies exploring USP1’s cancer inhibiting potential abound. In one of the latest study, the researchers used pharmacological inhibitors (ML323 and pimozide) and siRNA targeting USP1 to investigate the molecular mechanisms of USP1 inhibition in cancer cells. An elevated USP1 expression occurred in tumor tissues correlated with poor prognosis in renal cell carcinoma (RCC). USP1 also plays a role in stabilizing survivin, a protein inhibiting apoptosis and promoting cell survival, by enhancing survivin stabilization through removing ubiquitin tags. Inhibiting USP1 would downregulate survivin expression, indicating USP1 could be a potential chemotherapy target.
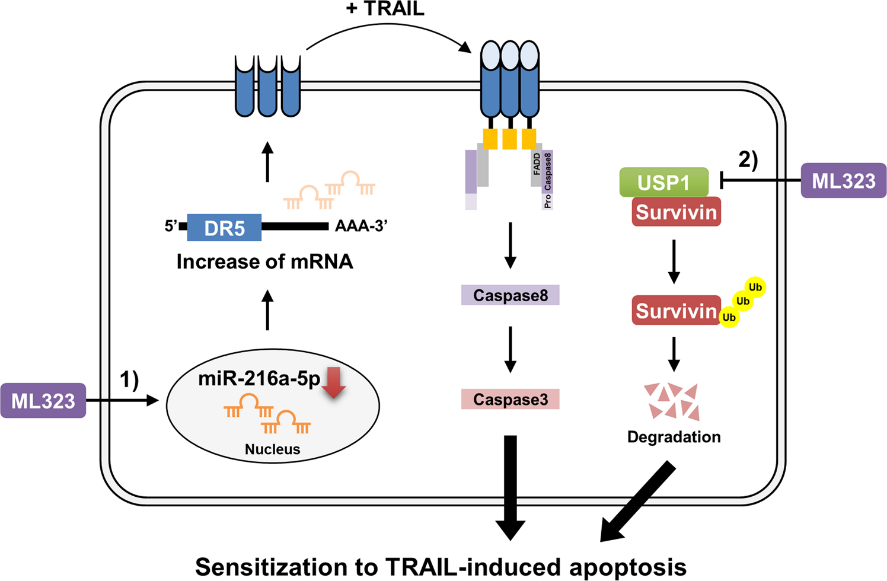
The same study also investigated the effects of ML323, a USP1 inhibitor, on cancer cells. ML323 upregulated DR5 expression by decreasing miR-216a-5p expression post-transcriptionally. DR5, inducing apoptosis, sensitized cancer cells to anticancer drugs upon upregulation.
A mouse xenograft model was used to test ML323’s USP1 inhibition effectiveness in vivo. The combined treatment with ML323 and TRAIL, an anticancer drug, significantly reduced tumor size by inducing survivin downregulation and DR5 upregulation. In the analysis that followed, USP1 and survivin protein expression showed a positive correlation, while miR-216a-5p and DR5 were inversely correlated.
The unusual binding mode
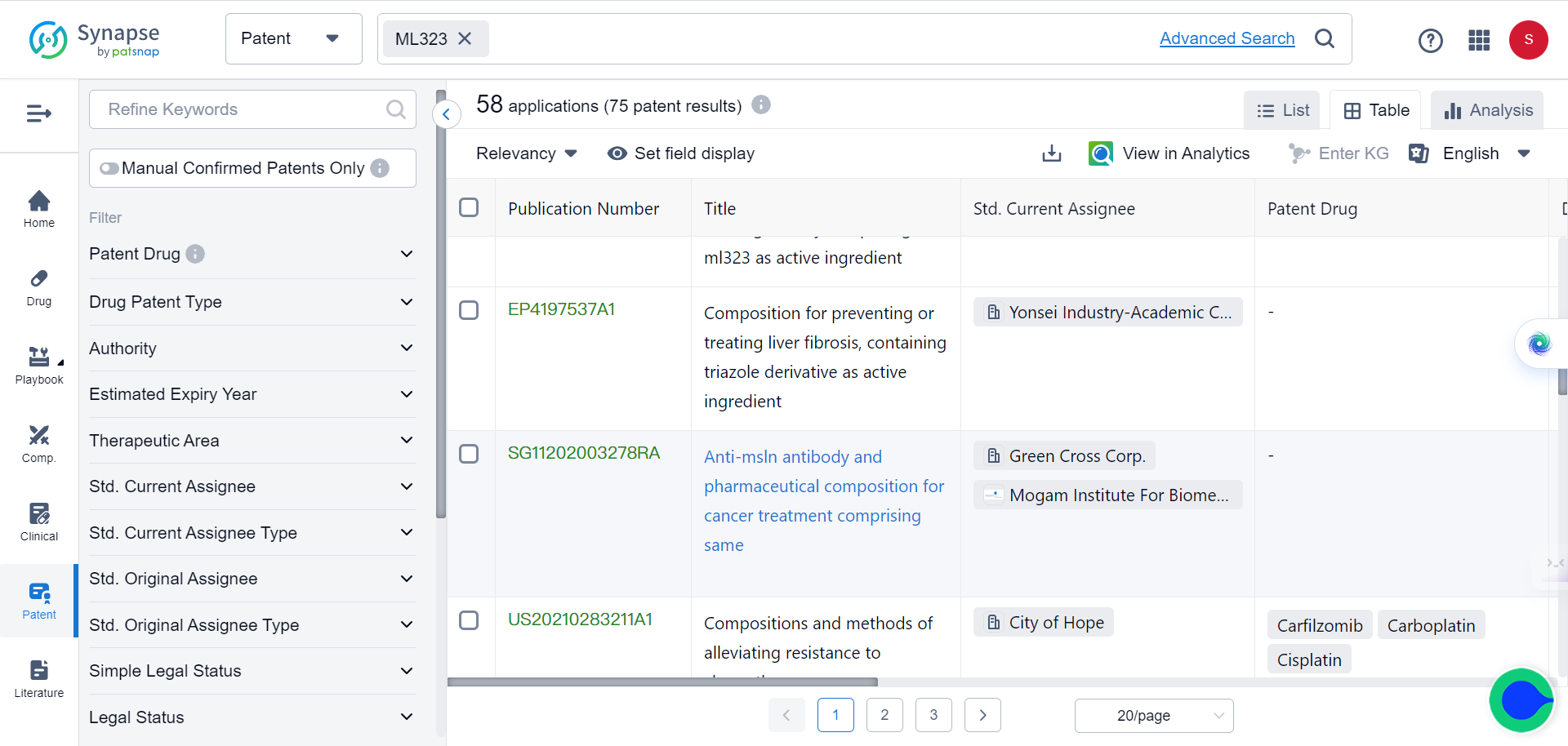
Although ML323 is a well-established inhibitor of USP1, with 75 related patents registered, its binding mode with USP1 has largely remained unknown, until recently.
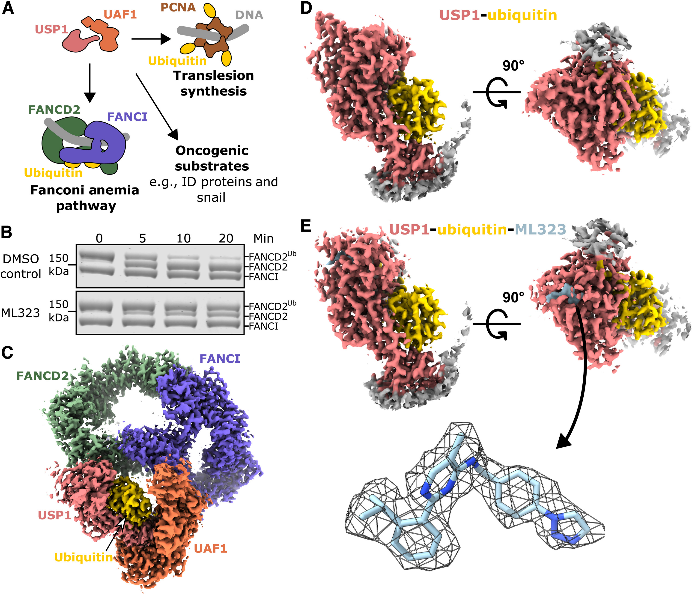
In a bid to understand how a USP1 inhibitor works, researchers from the University of Glasgow used cryo-electron microscopy (cryo-EM) to visualize an assembled enzyme-substrate-inhibitor complex of USP1 and the inhibitor ML323.
They tested ML323's ability to inhibit the deubiquitination of FANCI-FANCD2 by USP1-UAF1 in vitro. ML323 significantly reduced deubiquitination activity, showing inhibiting ability. Next, the USP1C90S-UAF1-FANCI-FANCD2Ub-dsDNA-ML323 complex was assembled and analyzed using cryo-EM, resulted in a 2.7 angstrom resolution reconstruction. Focused reconstruction of USP1-ubiquitin yielded 2.5 angstrom resolution structures of USP1 with and without ML323 bound.
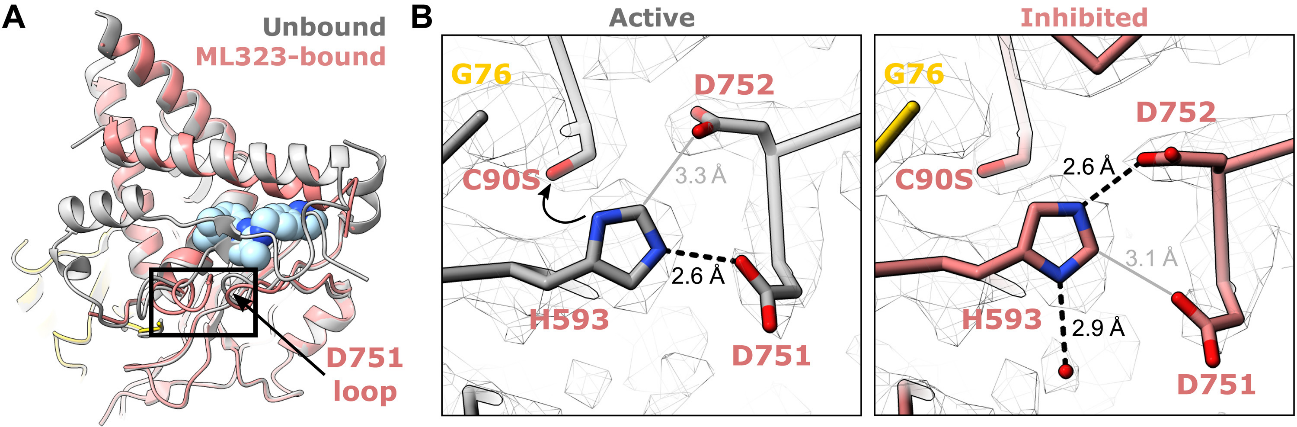
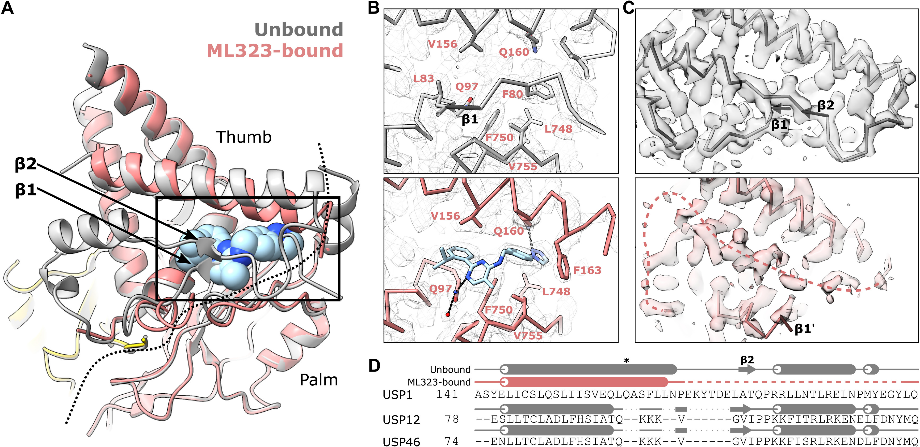
The data reveal that ML323 binds within USP1's hydrophobic core, replacing parts of the USP fold. This cryptic binding site lies between USP1's palm and thumb subdomains, about 9 angstroms from the active site cysteine. In this way, ML323 disrupts USP1's hydrophobic core, causing conformational changes that inhibit its function.
ML323 in action
ML323’s unconventional USP1 binding mode enables it to downregulate USP1 in colorectal cancer. In one recent study, researchers have discovered that USP1 can be polyubiquitinated and degraded by the proteasome system using colorectal cancer (CRC) cell lines. The team treated cells with the proteasome inhibitor MG132, which increased USP1 levels and ubiquitination, and prolonged its protein half-life. They found that the USP1 catalytic mutant C90S was not stabilized or ubiquitinated with MG132 treatment, indicating auto-ubiquitination depends on USP1's enzyme activity at this site.
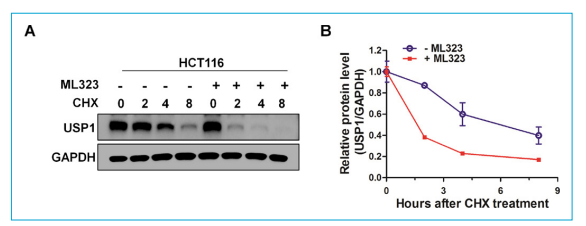
In addition, ML323 decreased USP1 protein levels in a dose and time-dependent manner in CRC cells. CHX chase assays indicated that ML323 shortened the half-life of USP1. Critically, MG132 reversed the USP1 downregulation induced by ML323, rescuing protein levels. This suggests that ML323 sends USP1 into the proteasome pathway. Further CHX chase assays confirmed that ML323-mediated destabilization of USP1 could be prevented by MG132. The caspase inhibitor Z-VAD did not affect ML323's impact on USP1, ruling out effects simply due to cell death. This result provides new insights into the regulation of USP1 and the action mechanism of ML323.
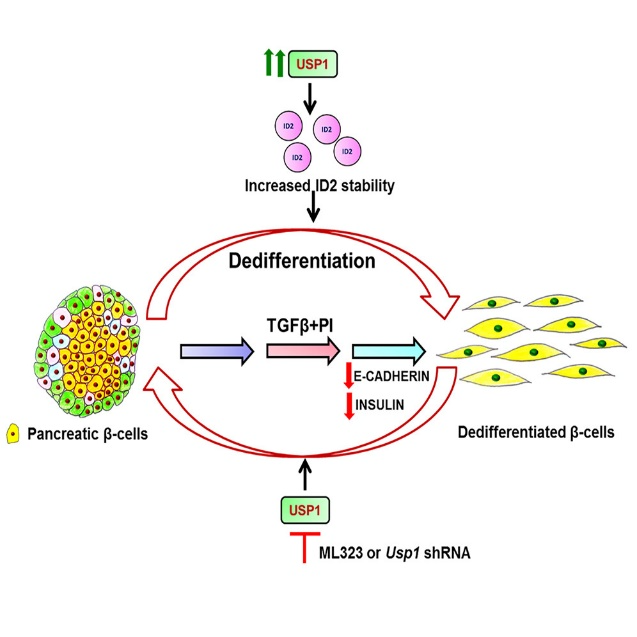
Other than treating cancers, ML323’s action on USP1 in animal models of diabetes has also been elucidated. The role of the USP1 in the dedifferentiation of pancreatic beta cells, which is a key contributor to the loss of insulin-secreting beta cells in diabetes, has been documented in previous studies. However, the treatment with ML323 inhibited dedifferentiation and restored the epithelial phenotype of beta cells. In the study published in iScience this May, researchers used ML323 at a concentration of 5 μM in vitro to inhibit USP1 activity in the MIN6 pancreatic beta cell line and found that ML323 treatment prevented TGFβ-induced dedifferentiation of beta cells, as evidenced by the maintenance of epithelial markers and the absence of mesenchymal markers.
In addition to in vitro studies, the efficacy of ML323 was also tested in an in vivo mouse model of STZ-induced dedifferentiation. The mice were treated with ML323 intraperitoneally at a dose of 20 mg/kg body weight for 10 days, which turned out to alleviate hyperglycemia in the STZ-induced dedifferentiation mouse model, suggesting that USP1 inhibition may have therapeutic potential in reducing beta cell loss during diabetes.
ML323 is not the only one that has caught researchers’ attention, other USP1 inhibitors have increasingly been placed under spotlight. ISM3091 is the latest example. This novel and selective USP1 inhibitor is reported to demonstrate potent anti-proliferation activity in the BRCA1m triple negative breast cancer (TNBC) cell line, MDA-MB-436, with a ~1500-fold selectivity for BRCAm vs BRCA WT.
In a nutshell, the discovery and development of USP1 inhibitors certainly open up new frontiers for the treatment of a variety of diseases, including cancer, diabetes, and potentially even more.
References:
1.Martin L., Rennie et al., Cryo-EM reveals a mechanism of USP1 inhibition through a cryptic binding site. Sci. Adv.8, eabq6353(2022).
2.Francis et al., Deubiquitinase USP1 influences the dedifferentiation of mouse pancreatic b-cells, iScience 26, 106771(2023).
3.Xu X, Mei X et al., The Deubiquitinating Enzyme USP1 is Auto-Ubiquitinated and Destabilized by ML323 in Colorectal Cancer Cells. EJMO 2023;7(2):174–179.
4.Woo S. M. et al., Inhibition of USP1 enhances anticancer drugs-induced cancer cell death through downregulation of survivin and miR-216a5p-mediated upregulation of DR5, Cell Death and Disease (2022) 13:821.