Breakthrough in Parkinson's Treatment: Positive Phase 3 Trial Results for Cerevel's Tavapadon
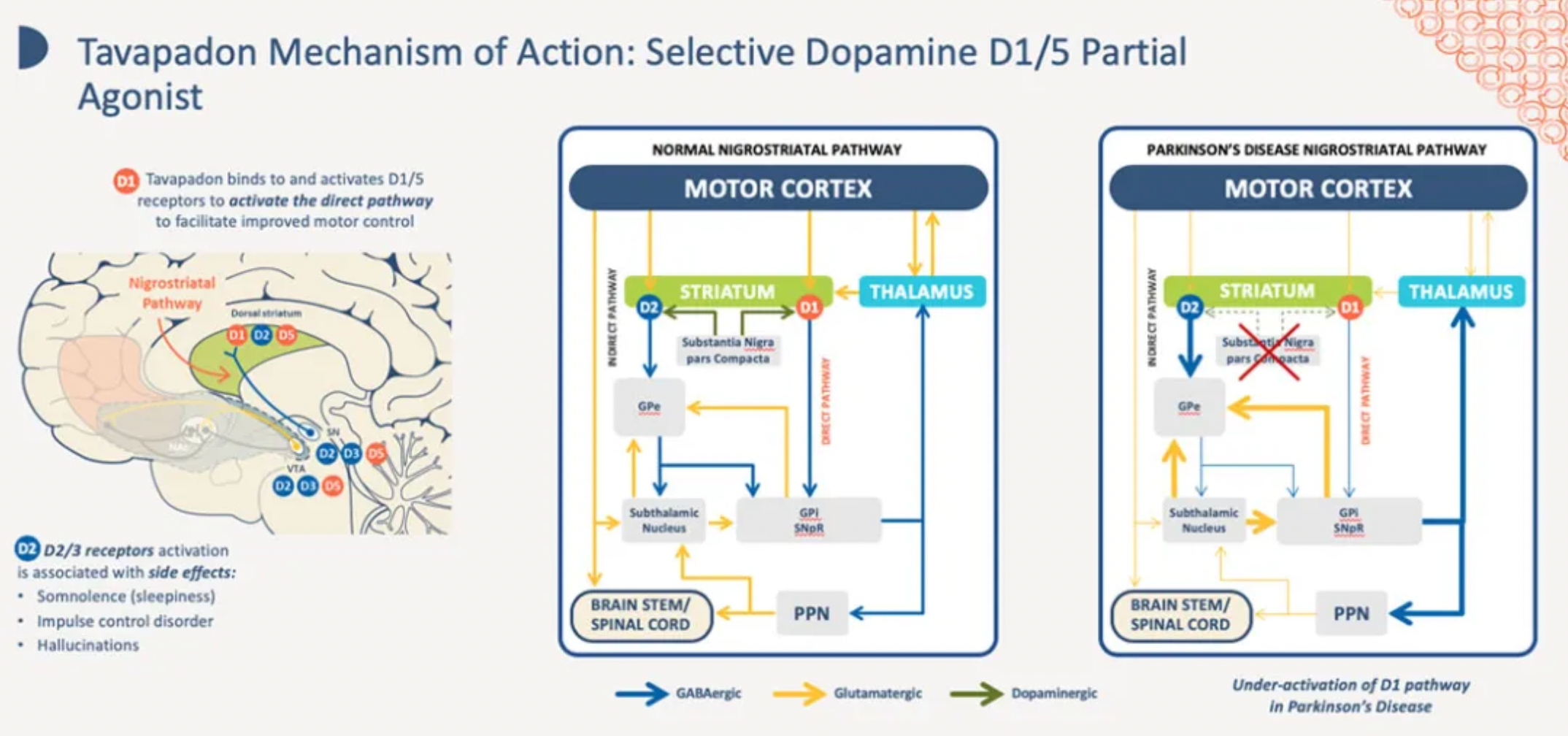
On April 18th, Cerevel announced the positive top-line results from its pivotal Phase 3 TEMPO-3 trial for tavapadon, the company's first and only D1/D5 receptor partial agonist, intended for the treatment of Parkinson's disease.
The TEMPO-3 trial assessed the efficacy, safety, and tolerability of tavapadon as a once-daily adjunctive therapy to levodopa in adult patients with Parkinson's disease. The trial demonstrated that, over a period of 27 weeks, patients treated with tavapadon experienced a statistically significant increase in total "ON time" without troublesome dyskinesia compared to those receiving placebo. Specifically, the clinical significance and statistical increase in "ON time" without troublesome dyskinesia was marked by an additional 1.1 hours (1.7 hours versus 0.6 hours, p<0.0001). The tavapadon treatment group also observed a statistically significant reduction in "OFF time," a key secondary endpoint.
These findings indicate that tavapadon may offer an appropriate balance of motor control, safety, and tolerability for patients with Parkinson's disease. Further results from the Phase 3 TEMPO-1 and TEMPO-2 trials of tavapadon as monotherapy are expected to be released in the second half of 2024.
Tavapadon (formerly known as PF-6649751) is a selective D1 and D5 receptor agonist originally developed by Pfizer. It is currently being developed by Cerevel for the clinical treatment of psychomotor disorders and Parkinson's disease.
About Cerevel
Cerevel Therapeutics was established at the end of 2018 in collaboration with Pfizer and Bain Capital. It is a clinical-stage biopharmaceutical company that combines a profound understanding of brain biology and neural circuitry with advanced chemistry and pharmacology of central nervous system receptors to discover and develop new therapies.
In December 2023, AbbVie announced plans to acquire Cerevel Therapeutics through a transformative transaction to enhance its pipeline strength in the field of neuroscience. This proposed acquisition is expected to bring a rich pipeline of top potential assets focused on unmet needs in psychiatry and neuroscience to AbbVie. It will complement AbbVie's current market portfolio and its emerging neuroscience pipeline, with the total transaction equity value estimated at approximately $8.7 billion.