Atom Bioscience begins enrolling U.S. participants in a worldwide Phase 2b/3 study of an experimental chronic gout therapy
Atom Bioscience, a biotechnology firm in the clinical stage, focused on innovative therapies for metabolic and inflammatory conditions, has initiated patient enrollment across the United States for a Phase 2b/3 study of ABP-671. This new treatment, an orally administered URAT1 inhibitor, targets chronic gout. To date, the trial has incorporated 36 locations spanning 17 states.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
A globally distributed multicenter trial, which is double-blind and randomized, plans to recruit 580 participants. The first phase of this trial focuses on assessing the safety and effectiveness of various doses and administration patterns of ABP-671, comparing these to either placebo or allopurinol, which is presently the preferred treatment for gout management in the U.S. The second phase of the experiment will examine selected dosing protocols of ABP-671 from the first part on a different group of patients not previously involved in the initial phase.
Gout represents a commonly encountered form of inflammatory arthritis, triggered by prolonged hyperuricemia—a condition where serum uric acid levels exceed 7 mg/dL.
The impact of gout on individual well-being is significant, elevating risks such as sudden cardiac death. The currently available medications for chronic gout are often inadequate in terms of efficacy and carry substantial risks, including potential for kidney failure, sudden cardiac death, and severe liver toxicity.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
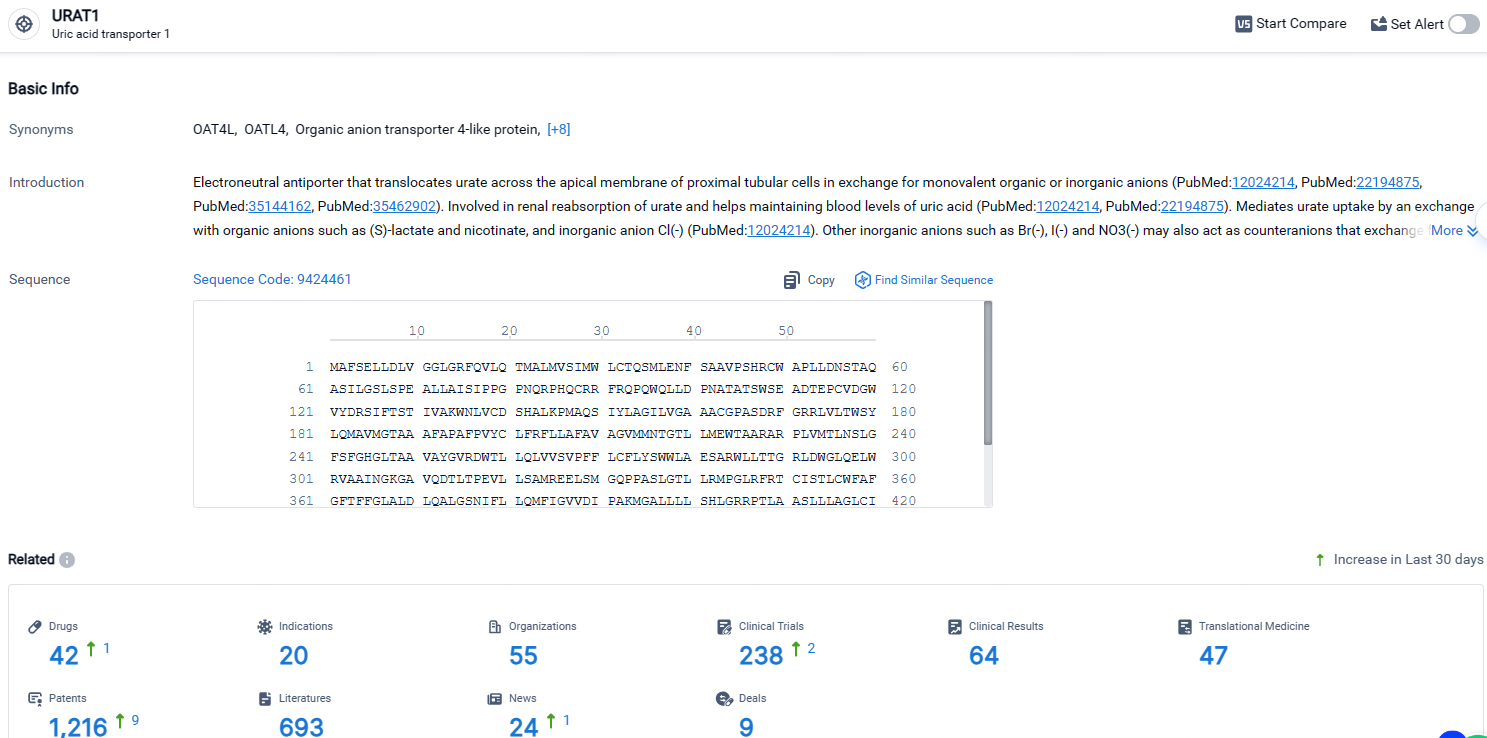
According to the data provided by the Synapse Database, As of April 28, 2024, there are 42 investigational drugs for the URAT1 target, including 20 indications, 55 R&D institutions involved, with related clinical trials reaching 238, and as many as 1216 patents.
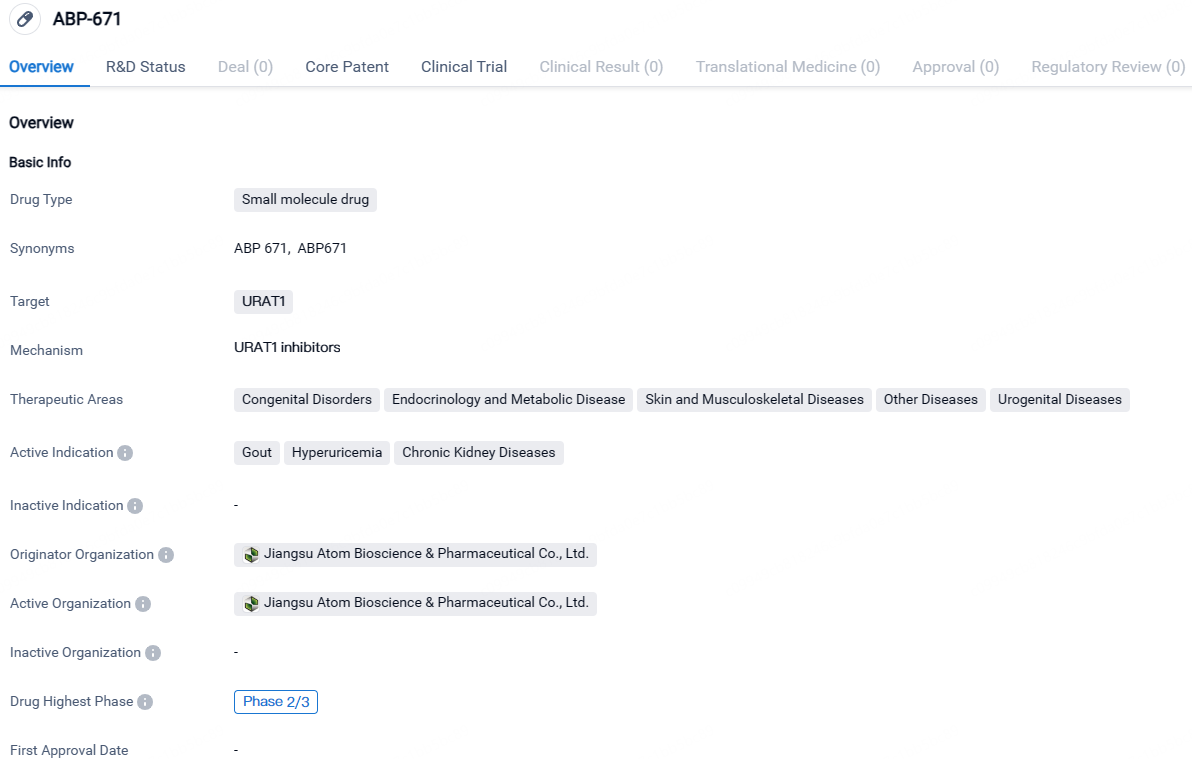
ABP-671 is a small molecule drug that targets URAT1 and is being developed by Jiangsu Atom Bioscience & Pharmaceutical Co., Ltd. It is currently in Phase 2/3 of clinical development and shows potential for treating gout, hyperuricemia, and chronic kidney diseases. The drug's therapeutic areas encompass a wide range of conditions, indicating its potential for broader applications in the field of biomedicine.