Brenig Therapeutics Initiates First Human Trial for Parkinson's Drug BT-267
Brenig Therapeutics has declared the commencement of a first-in-human clinical study for BT-267, a leading LRRK2 inhibitor created to serve as a potential disease-modifying therapy for both idiopathic and LRRK2-related Parkinson’s disease. The progression of Brenig’s clinical initiatives is bolstered by a recent funding round of $65 million, spearheaded by New Enterprise Associates.
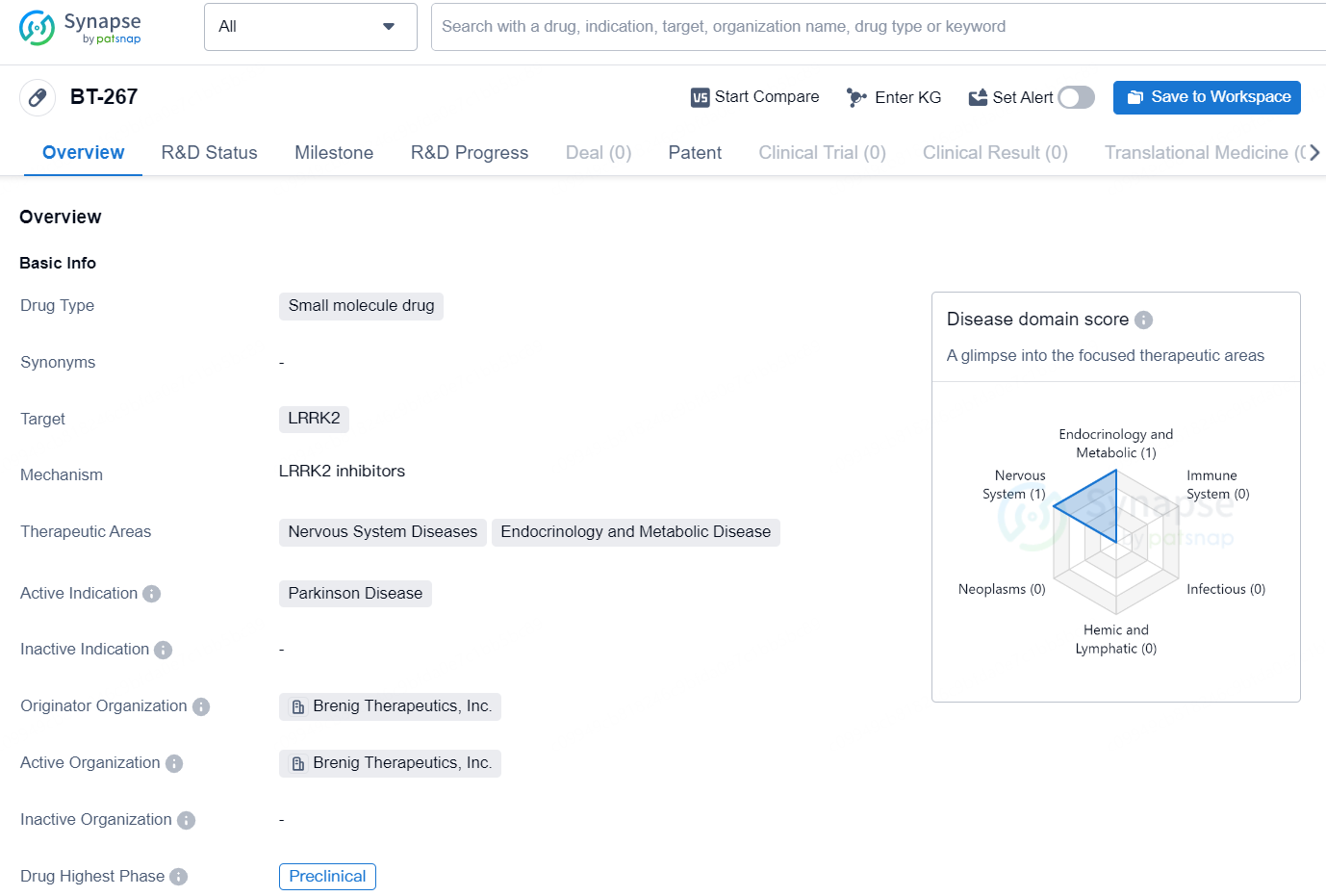
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The clinical trial commenced in November 2024, administering doses to healthy volunteers to assess the safety and tolerability of BT-267. Upon completion of this initial evaluation, proof-of-concept studies are set to follow involving patients diagnosed with idiopathic Parkinson’s disease.
Preclinical findings highlight BT-267’s potential as a leading LRRK2 inhibitor, illustrating an excellent safety profile with minimal or no apparent morphological changes in the lungs or kidneys even at the highest doses tested in GLP toxicology studies. Additionally, it showcases remarkable pharmacokinetics, particularly a high unbound ratio of cerebrospinal fluid (CSF) to plasma.
BT-267 is supported by state-of-the-art computer-aided drug design, AI/ML-driven computational pharmacology, biomarker models, and the structural biology proficiency of its collaborating partner, Expert Systems Accelerator. Brenig’s innovative approach to predictive biomarkers may enhance the positioning of BT-267 as a potential disease-modifying treatment for Parkinson’s disease, including idiopathic forms that do not have identified genetic causes.
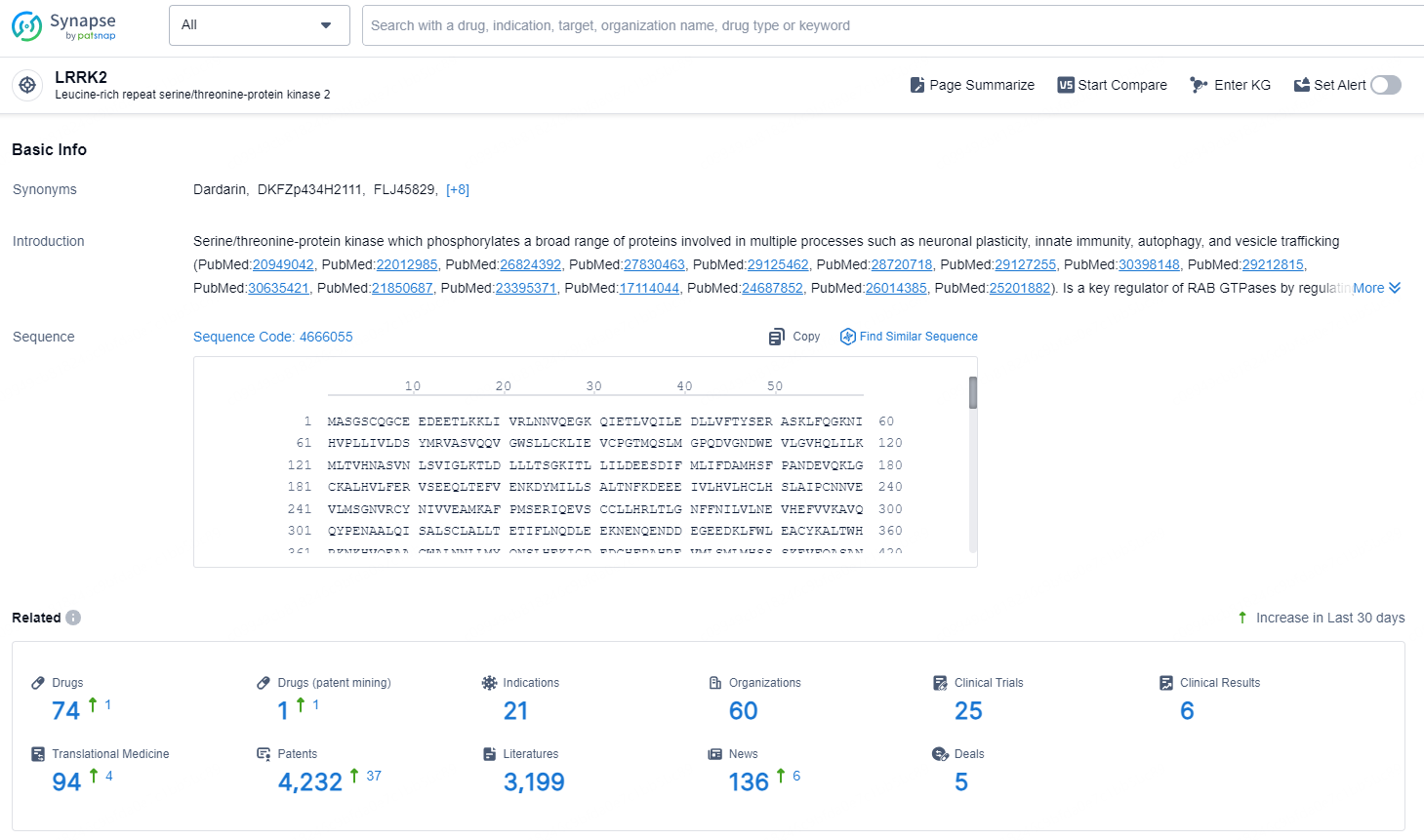
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of December 2, 2024, there are 74 investigational drugs for the LRRK2 target, including 21 indications, 60 R&D institutions involved, with related clinical trials reaching 25, and as many as 4232 patents.
The drug BT-267 is a small molecule drug targeting LRRK2, primarily intended for the treatment of Parkinson's Disease. It falls under the therapeutic areas of Nervous System Diseases and Endocrinology and Metabolic Disease. The drug is currently in the preclinical phase, indicating that it has not yet progressed to clinical trials with human subjects.