BridgeBio and Resilience declares a collaboration aiming to progress BBP-631, BBP-812
BridgeBio Pharma Inc., a company primarily dealing with commercial-stage biopharmaceuticals for genetic diseases and cancers, along with National Resilience Inc., a biomanufacturing firm with a tech-centric approach towards expanding accessibility to intricate medications, publicized a strategic alliance. This partnership aims to progress and produce BBP-812 and BBP-631; the investigational adeno-associated virus 9 and 5 gene therapy for the treatment of Canavan disease and congenital adrenal hyperplasia respectively.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
In gene therapy, both companies have created a new manufacturing and combined benefit scheme to promote these therapies, focusing on sustainability and monetary efficacy. As per the agreement, manufacturing protocol of BridgeBio's primary AAV-based gene therapy candidates will be handed over to Resilience's hub of gene therapy facilities.
Under an innovative model of cost and risk management, Resilience will lend its manufacturing services and will gain subsequent development and sanctioning milestones, along with low to medium single-digit royalties on BBP-631 and BBP-812. Resilience will cater to the continuous clinical manufacturing demand, and if successful, will stand as the chief commercial manufacturer for both programs.
Resilience's CEO, Rahul Singhvi, Sc.D., stated, “Our tie-up with BridgeBio is aimed at speeding the creation of inventive therapeutic alternatives for patients who need it. We're thrilled about partnering with a leader in gene therapy and rare diseases and we're motivated by their drive to bring remedies to patients."
In addition to BBP-812 and BBP-631, Resilience will also manufacture upcoming clinical projects for BridgeBio’s gene therapy range. This pact will diminish production uncertainties for these programs and will supposedly assist in BridgeBio’s quicker execution of gene therapies.
"Working together with Resilience's proficient and knowledgeable team on our gene therapies' production is a great opportunity for us," expressed Neil Kumar, Ph.D., BridgeBio's founder, and CEO. "We aspire for this to speed up our course to benefit a maximum number of patients at the earliest."
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
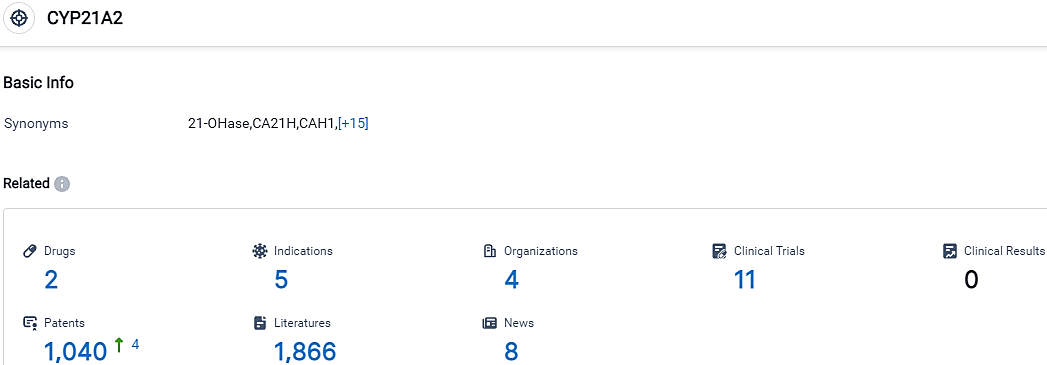
According to the data provided by the Synapse Database, As of October 14, 2023, there are 2 investigational drugs for the CYP21A2 target, including 5 indications, 4 R&D institutions involved, with related clinical trials reaching 11,and as many as 1040 patents.
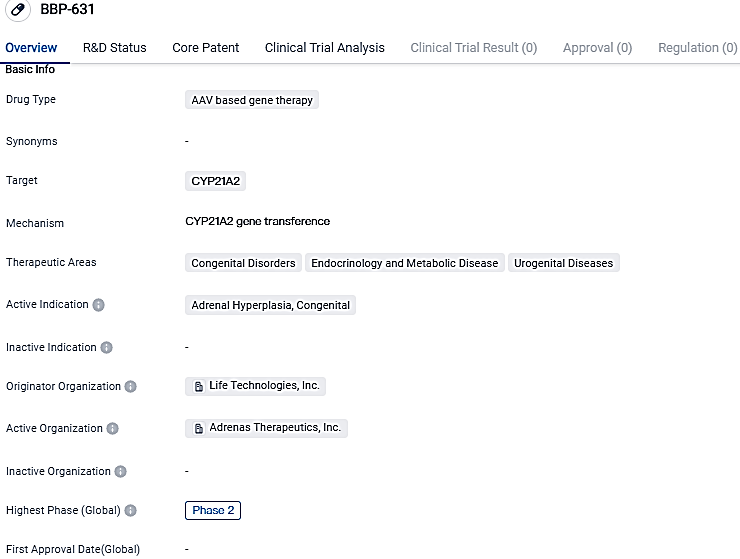
BBP-631 is designed to deliver a functional copy of the 21-hydroxylase gene. If successful, BBP-631 may restore the body’s hormone and steroid balance by enabling people with CAH to naturally make their own cortisol and aldosterone. It could also allow for people with CAH to eliminate or significantly reduce their daily glucocorticoid or mineralocorticoid doses, which is the current standard of care for patients.