Bristol Myers Squibb Expands Portfolio with Purchase of Leading Radiopharmaceutical Company RayzeBio
Pharmaceutical giant Bristol Myers Squibb has entered into a binding merger contract to purchase biotech firm RayzeBio, Inc. The deal sets the purchase price at $62.50 for each share, entirely in cash transactions, which sums up to an overall value of around $4.1 billion in equity. When considering the cash holdings that Bristol Myers Squibb is expected to inherit, the net acquisition cost stands at about $3.6 billion. This strategic move has received full backing from the governing bodies of both corporations, with unanimous consent achieved from the Board of Directors at Bristol Myers Squibb as well as RayzeBio.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Operating in the domain of clinical-stage radiopharmaceutical treatments, RayzeBio is positioned at the forefront of innovation involving actinium-based RPTs. The company is currently advancing a suite of unique drug development initiatives which may lead the market as first-in-class and best-in-class offerings. Their developmental thrust is primarily directed toward solid tumor therapies, with specific applications for gastroenteropancreatic neuroendocrine tumors, small cell lung cancer, hepatocellular carcinoma, and several other forms of cancer.
"Joining forces with RayzeBio represents a significant step in broadening our cancer treatment portfolio, introducing a unique technology and collection of drug candidates. This move is poised to underpin our expansion in the latter half of this decade and into the future," commented Christopher Boerner, Ph.D., the Chief Executive Officer at Bristol Myers Squibb.
Expressing optimism about the alliance, Ken Song, M.D., the President and CEO of RayzeBio, stated, "The established expertise of Bristol Myers Squibb within the oncology sector and their profound skills in growing, commercializing, and manufacturing on an international scale positions them as the premier collaborator for RayzeBio during this pivotal phase. I am eager to witness the achievements our team will realize within the Bristol Myers Squibb environment."
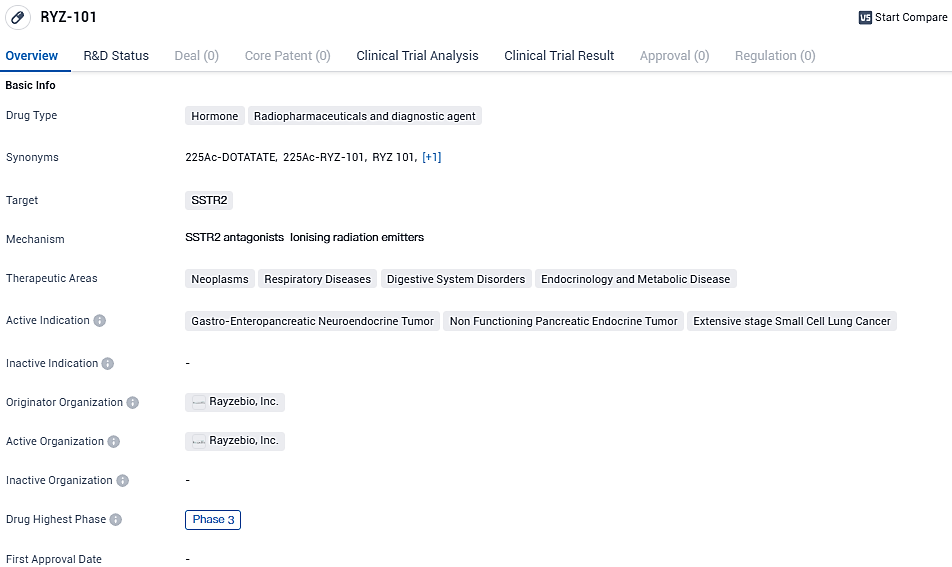
A primary focus of RayzeBio's lineup is RYZ101, designed to target somatostatin receptor 2 (SSTR2), which finds increased expression in GEP-NETs and extensive stage small cell lung cancer. There is an ongoing Phase 3 clinical trial actively recruiting subjects in order to assess RYZ101's effectiveness in patients with detectable SSTR-positive GEP-NETs, who have already undergone treatments with lutetium-177 tagged somatostatin analogs.
RayzeBio has recently shared preliminary outcomes from the ACTION-1 Phase 1b clinical trial, indicating promising signs of efficacy and safety. Another Phase 1b trial is in progress to determine the viability of RYZ101 as an initial treatment modality for ES-SCLC, in conjunction with the current standard treatment protocols.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
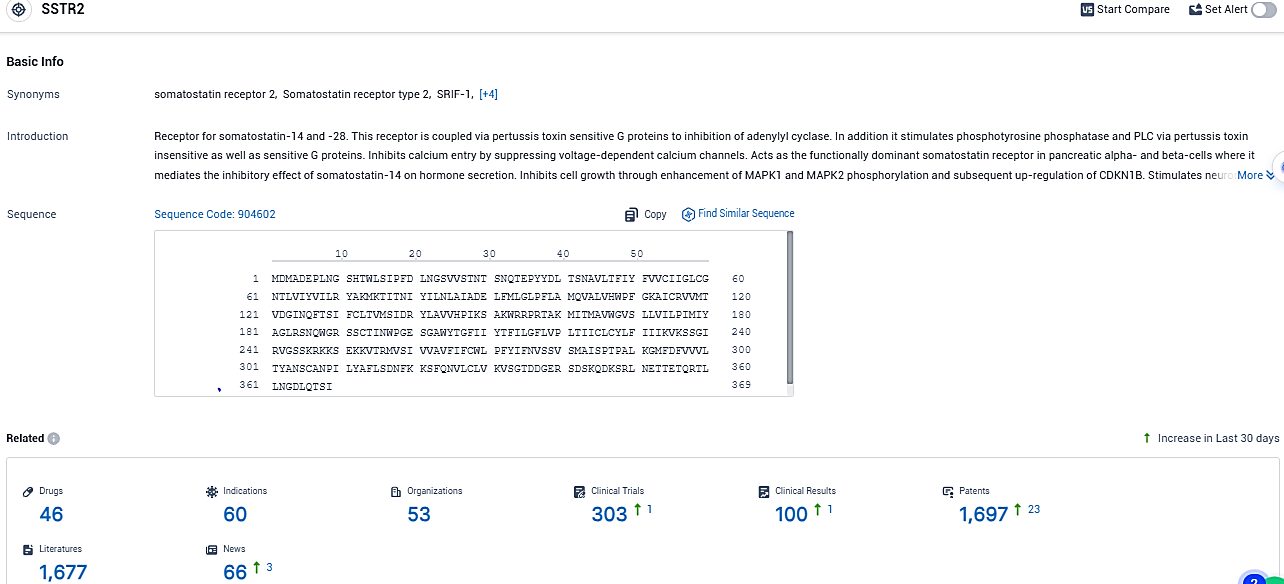
According to the data provided by the Synapse Database, As of December 30, 2023, there are 46 investigational drugs for the SSTR2 target, including 60 indications, 53 R&D institutions involved, with related clinical trials reaching 303, and as many as 1697 patents.
RYZ-101 is a hormone, radiopharmaceutical, and diagnostic agent that targets SSTR2. It has shown potential in treating various neoplasms, respiratory diseases, digestive system disorders, and endocrinology and metabolic diseases.