Research findings on Chugai's innovative multispecific antibody DONQ52, aimed at treating Celiac Disease
Chugai Pharmaceutical Co., Ltd. has released an announcement indicating that the findings from preclinical studies on the multifunctional antibody named DONQ52 have been documented in the prestigious journal, Nature Communications. The innovative antibody DONQ52 is an original discovery by Chugai, and it is currently being evaluated in a Phase I clinical trial targeting patients with celiac disease.
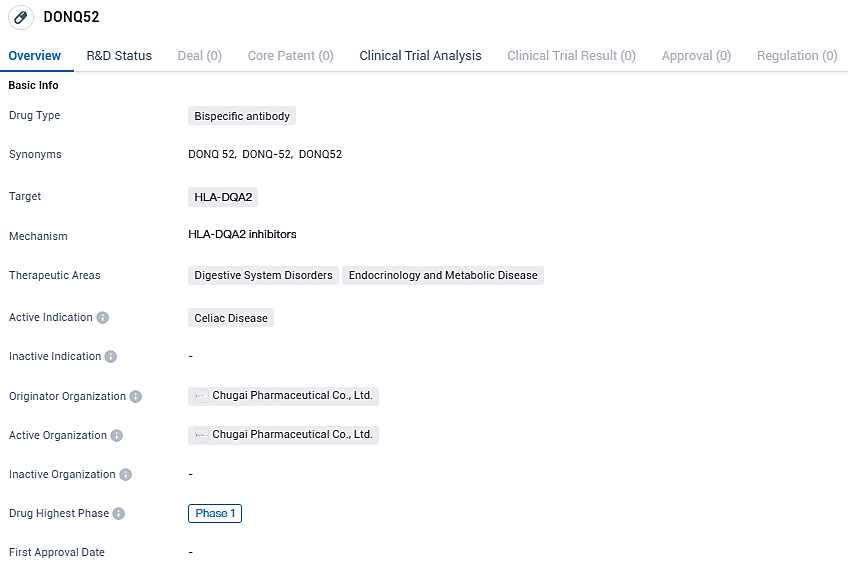
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Nature Communications, a premier open-access journal spanning various disciplines within science, is published by Nature Publishing Group. This journal is renowned for disseminating high-caliber research from a diverse spectrum including life sciences, medicine, social studies, physics, chemistry, and Earth sciences. Recent studies have highlighted the efficacy of a compound named DONQ52 in modulating the immune system's reaction to gluten among individuals with celiac disease, a disorder for which there is currently no sanctioned treatment.
Chugai's head, Dr. Osamu Okuda, expressed his satisfaction with their latest discovery being featured in Nature Communications. He remarked, “Our foundational research on the multispecific antibody DONQ52, crafted in our labs, has reached a broad audience thanks to this publication. Despite challenging obstacles that have delayed its application, our exploratory research illustrates that curtailing the interaction between HLA and T-cells could represent a promising strategy for treating celiac disease."
Dr. Okuda conveyed excitement about the ongoing phase I trials, anticipating that DONQ52 will exhibit an exceptional safety profile along with the capability to dampen the immune response against gluten in a targeted manner. "It is our hope that this agent will emerge as a viable treatment for those suffering from celiac disease. Leveraging cutting-edge antibody engineering, our aim is to pioneer a new wave of antibodies that surpass the limitations of traditional ones," Dr. Okuda continued.
Chugai's investigation has identified DONQ52, a multispecific antibody targeting the HLADQ2.5/gluten peptide complex, as a potential therapeutic candidate for celiac disease—a genetic autoimmune disorder lacking approved treatment options. Through widespread inhibition of the complex's binding to the T-cell receptor, DONQ52 aims to directly thwart T-cell activation, addressing the primary driver of celiac disease's pathology.
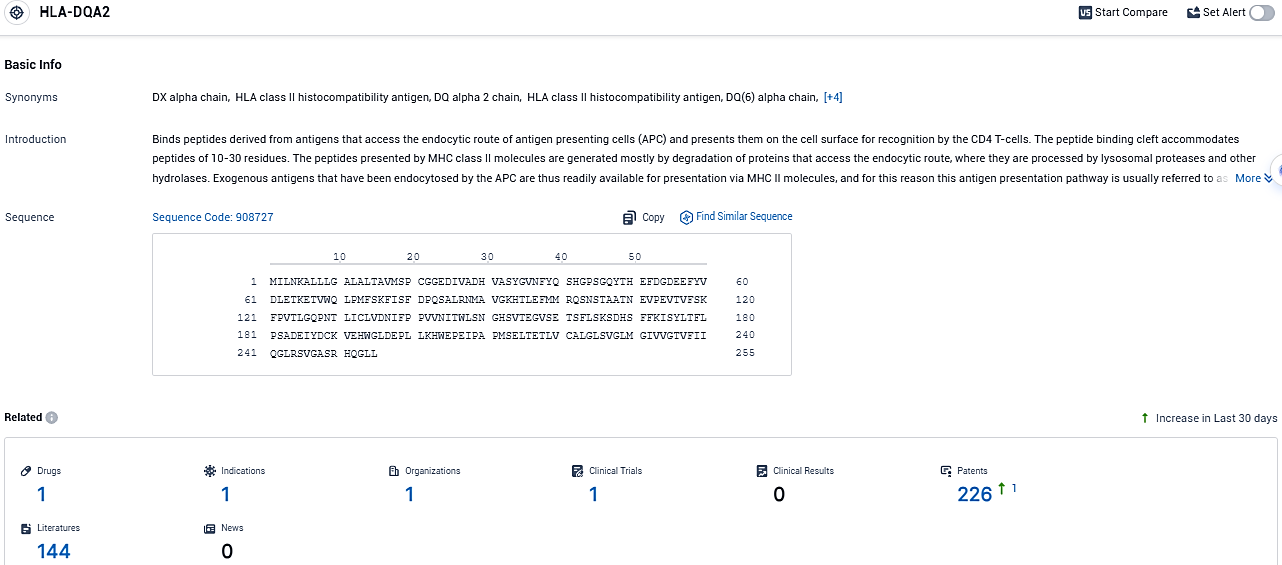
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of December 30, 2023, there are 1 investigational drugs for the HLA-DQA2 target, including 1 indications, 1 R&D institutions involved, with related clinical trials reaching 1, and as many as 226 patents.
DONQ52 is currently in Phase 1 of clinical trials and targets HLA-DQA2, a gene involved in the immune response. While the drug's therapeutic areas include Digestive System Disorders and Endocrinology and Metabolic Disease, its primary focus is on Celiac Disease. Further research and clinical trials will be needed to determine the efficacy and safety of DONQ52 in treating this condition.