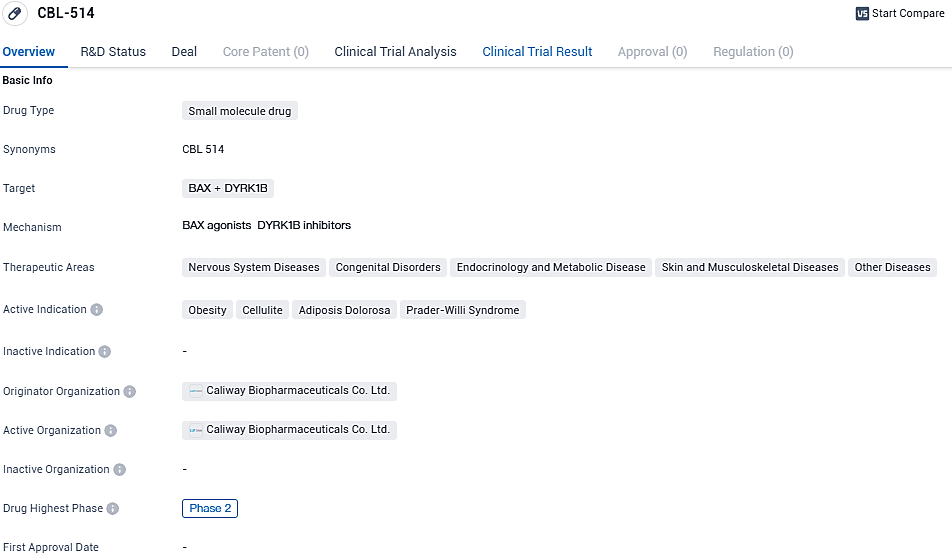
Caliway Secures Over $100M for Phase 3 Trial of Fat-Reduction Drug CBL-514
Caliway Biopharmaceuticals has disclosed the completion of a highly successful financing round, obtaining in excess of $100 million through the release of 8 million additional shares to investors. These fresh resources are earmarked for propelling the progression of their primary product candidate, CBL-514. This signature compound, a small-molecule injectable medication, is designed to diminish localized fat deposits beneath the skin in targeted treatment zones.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The allocation of the funding is directed towards comprehensive Pivotal Phase 3 trials across multiple countries and centers aiming at the reduction of subcutaneous fat without surgery, along with a Phase 2b trial of CBL-514 for the treatment of Dercum's disease versus a placebo.
"We are excited to report that the outcomes of the CBL-0202 Phase 2 trial reveal CBL-514 to be notably effective in reducing subcutaneous fat. On average, a reduction exceeding 300 mL in the targeted zone was observed when compared to the placebo. Significantly, 85.7% and 76.2% of subjects experienced a loss of at least 150 mL and 200 mL respectively of subcutaneous fat in the treated regions after CBL-514 intervention(s)," expressed Vivian Ling, CEO of Caliway. "Thanks to the recent influx of capital, we are eager to advance CBL-514 through successive phases of its clinical progression, striving to meet the critical healthcare gaps in the management of both subcutaneous fat and Dercum's disease."
Caliway has initiated the regulatory submission process for the CBL-514 Pivotal Phase 3 study focused on subcutaneous fat diminishment with Australia's Bellberry HREC. Sequentially, submissions for the Pivotal Phase 3 study IND are in preparation for the U.S. FDA, EMA, and regulatory bodies in various nations.
Furthermore, December 2023, marked the submission of the IND for the Phase 2b trial of CBL-514 regarding Dercum's disease to the U.S. FDA. This particular study is poised to rigorously assess the effectiveness, safety profile, and tolerability of CBL-514 in the context of Dercum's disease treatment, relative to a placebo.
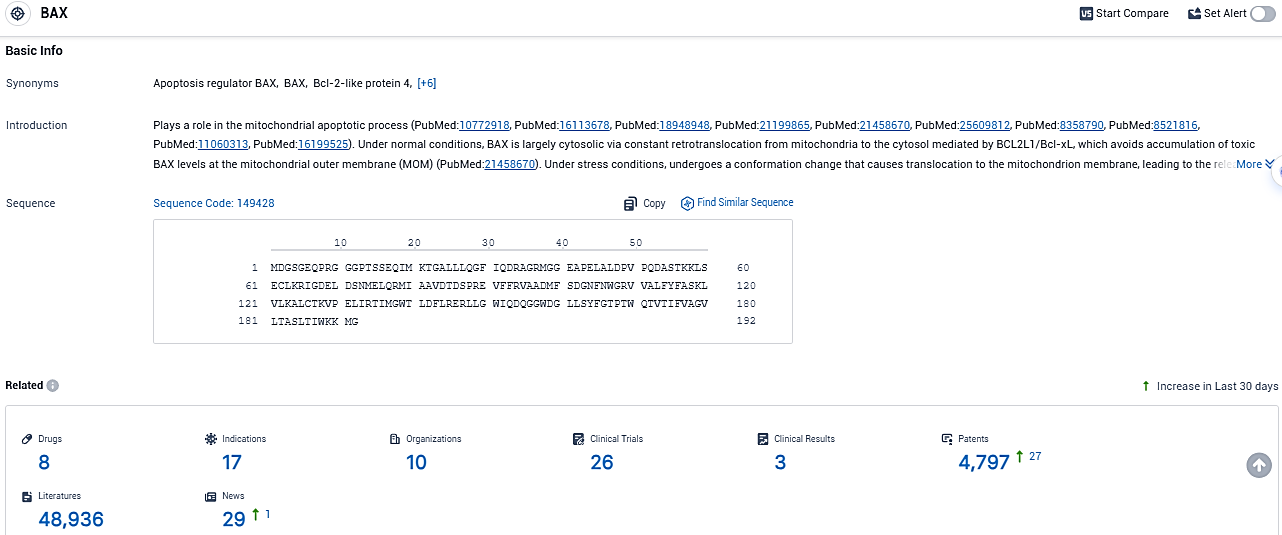
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of December 27, 2023, there are 8 investigational drugs for the BAX target, including 17 indications, 10 R&D institutions involved, with related clinical trials reaching 26, and as many as 4797 patents.
CBL-514, a potentially first-in-class small-molecule drug, is an injection lipolysis drug that can induce adipocyte apoptosis and lipolysis to reduce subcutaneous adiposity in treatment areas in animal studies without causing any systematic side effects on the central nervous system, cardiovascular system, and respiratory system. Caliway's nonclinical studies showed that CBL-514 upregulates the apoptosis mediators caspase 3 and Bax/Bcl-2 ratio, and then induces dose-dependent adipocyte apoptosis in vivo and in vitro.