Capmatinib Hydrochloride: brief review of its R&D progress and the clinical result in 2023 ESMO
The clinical trial for Capmatinib Hydrochloride took center stage at the 2023 ESMO Congress, demonstrating its potential to treat aNSCLC.
Capmatinib Hydrochloride's R&D Progress
Capmatinib Hydrochloride is a small molecule drug that targets c-Met, a protein involved in cell growth and survival. It has shown potential therapeutic benefits in various therapeutic areas including neoplasms, skin and musculoskeletal diseases, digestive system disorders, nervous system diseases, urogenital diseases, and respiratory diseases.
According to the Patsnap Synapse, Capmatinib Hydrochloride has reached the highest phase of development, which is approved globally. And the clinical trial areas for Capmatinib Hydrochloride are primarily in the United States, China, and United Kingdom. The key indication is Non-Small Cell Lung Cancer.
Detailed Clinical Result of Capmatinib Hydrochloride
The randomized, parallel assignment, open-label, randomized, phase 3 trial (NCT04427072) was aimed to evacuate the available efficacy and safety of cap vs docetaxel (doc) from GeoMETry-III trial.

In this study, Eligible pts included EGFR wild type, ALK rearrangement−negative, stage IIIB/IIIC or IV METex14–mutated NSCLC who have progressed on 1/2L of systemic therapy. Primary and key secondary endpoints were progression-free survival (PFS) and overall response rate (ORR) by blinded independent review committee (BIRC) assessment per RECIST v1.1, respectively. Although 90 pts were planned to be included in this study, due to slow enrollment, the study was terminated early with 22 patients enrolled.
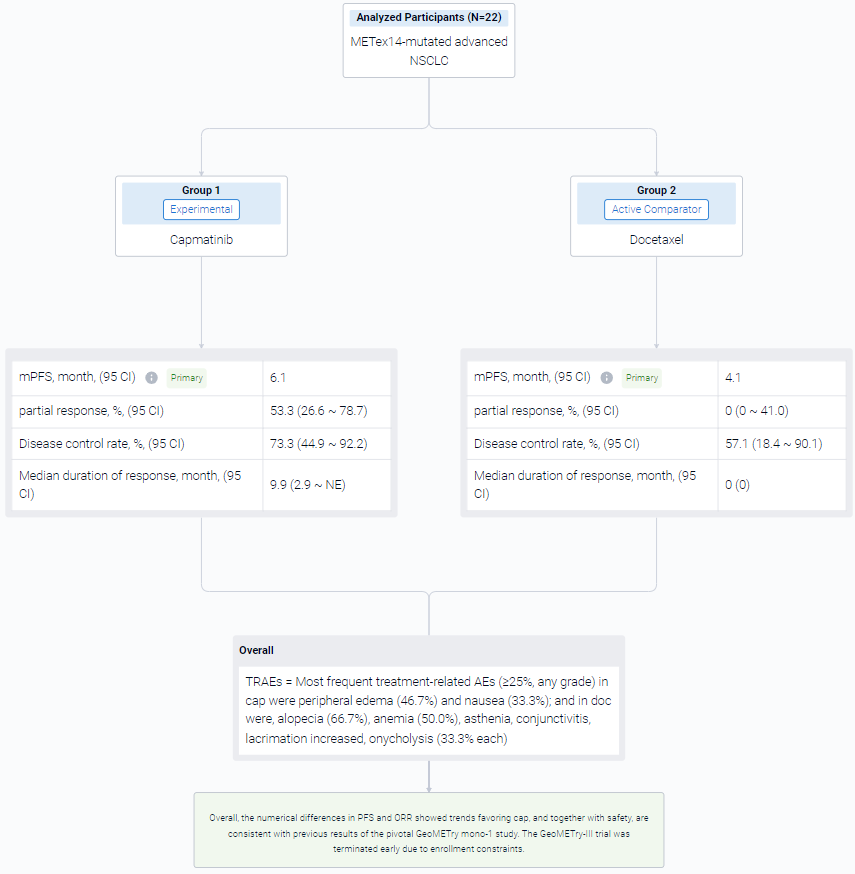
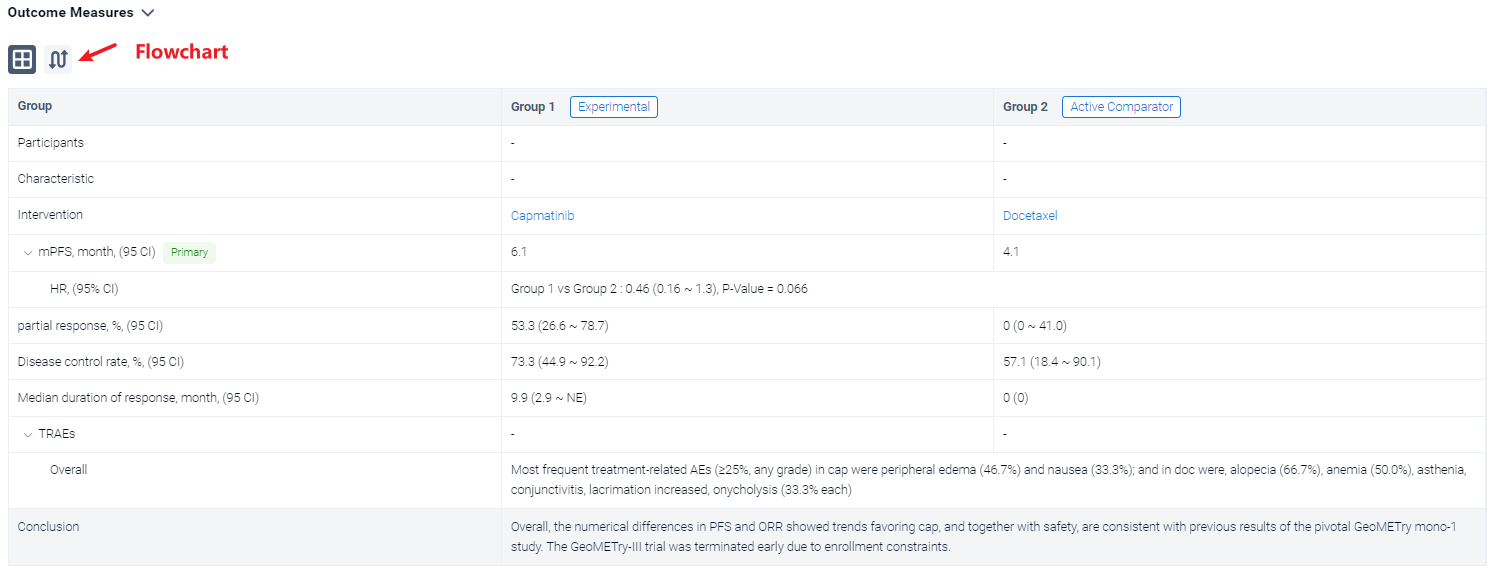
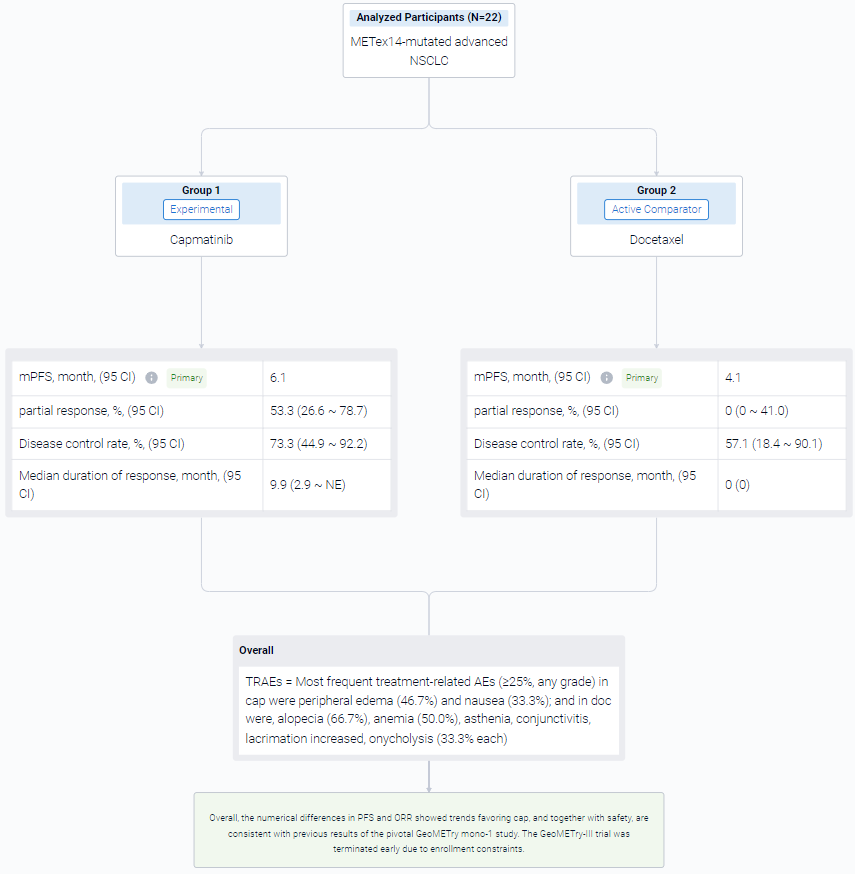
The result showed that as of Feb 15, 2023, data cutoff, 22 pts were randomized 2:1 to cap (n = 15) or doc (n = 7) arms. Baseline characteristics were generally comparable between arms. The observed benefit with cap vs doc in median PFS was not statistically significant: 6.1 months vs 4.1 months; HR, 0.46; 95% CI 0.16-1.3; P = 0.066. Of 15 pts in cap arm, 8 had partial response (53.3%, 95% CI 26.6-78.7) vs none in doc arm (95% CI 0-41.0). Disease control rate was 73.3%; 95% CI 44.9-92.2 vs 57.1%; 95% CI 18.4-90.1, respectively. Median duration of response in cap was 9.9 months; 95% CI 2.9-NE. Five of 6 doc-treated pts crossed over to cap (at 1.4, 1.9, 6.0, 6.3, and 13.7 months from doc start due to progression), which confounded the overall survival results. No new safety signals were observed for cap. Most frequent treatment-related AEs (≥25%, any grade) in cap were peripheral edema (46.7%) and nausea (33.3%); and in doc were, alopecia (66.7%), anemia (50.0%), asthenia, conjunctivitis, lacrimation increased, onycholysis (33.3% each).
It can be concluded that the numerical differences in PFS and ORR showed trends favoring cap, and together with safety, are consistent with previous results of the pivotal GeoMETry mono-1 study. The GeoMETry-III trial was terminated early due to enrollment constraints.
How to Easily View the Clinical Results Using Synapse Database?
If you want to know the other clinical results of popular conferences, please lick on the “Clinical Results” on the homepage of Patsnap Synapse, which provides multi-dimensional screening and filtering of drugs, indications, targets, companies, result evaluation, release date, popular conferences, etc. to help you quickly locate the data you need.
Select the clinical meeting you are interested in, such as ESMO. In the results, you can quickly locate the data you want to view by indication, phase and drug name.
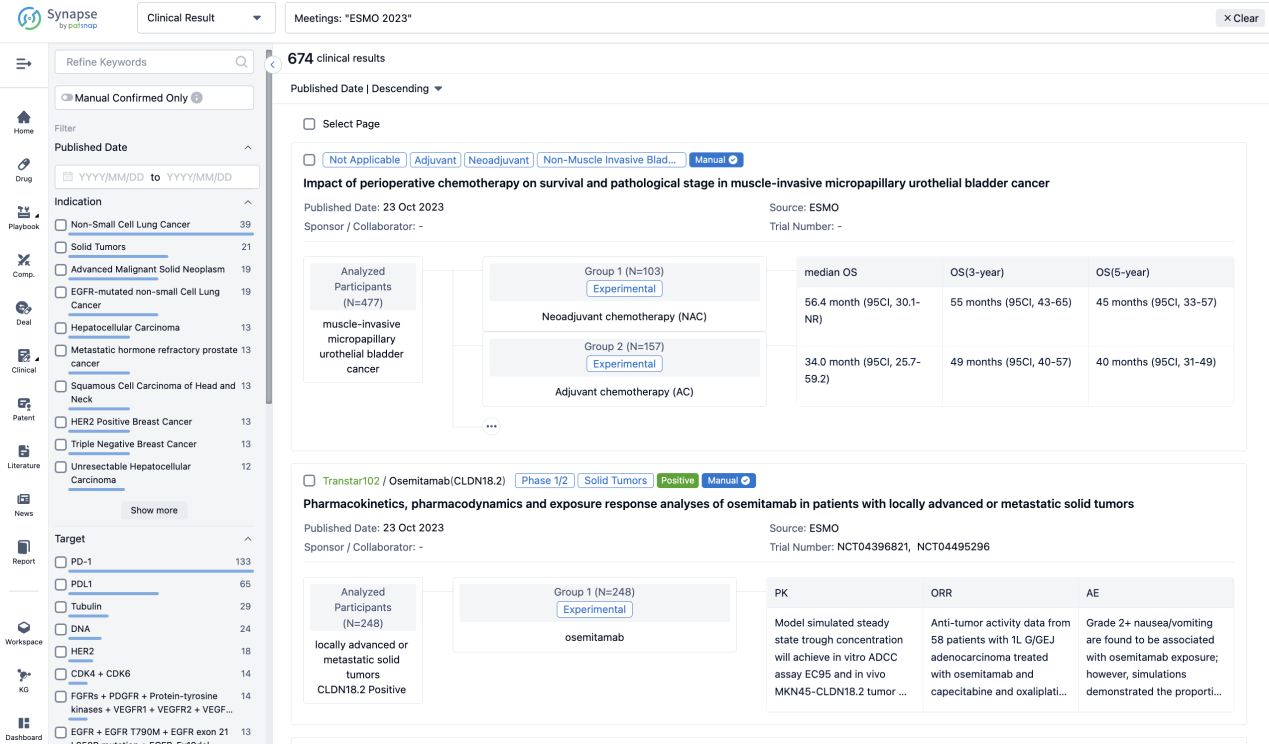
A single result clearly shows important information such as registration number, phase, indication, Sponsor/Collaborator, biomarker, Trial number, dosing regimen and more.
If you would like to view more information about this result, you can go to the result detail page by clicking on the title.
Above the headings, we provide the original source of the outcome data. The basic information is supplemented with more information beyond the list, such as company, study. design, etc.

In the important Outcome Measures section, we provide both list and flowchart forms, which are convenient for you to overview the comparison group information and core indicator data.
Finally, if you need to download these results, you can conveniently check the check boxes on the left side of the list, or directly click the "Export" button to download the data for personalized analysis and file sharing.
Click on the image below to embark on a brand new journey of drug discovery!