ADC drugs worth paying attention to in Clinical Phase III/II/I
Without a doubt, ADC (Antibody Drug Conjugates) is one of the hottest fields in recent years. In 2022, the global sales exceeded 7 billion US dollars, and the sales of these drugs in the first half of this year have already exceeded 5 billion US dollars. It is expected to break the 10 billion dollar mark for the full year. Currently, 15 ADCs have been approved for marketing.
Hundreds of ADCs are under investigation worldwide, and in pursuit of differentiation and increased competitiveness, an increasing number of targets are being developed, including popular targets such as HER2, Trop-2, EGFR, and emerging targets like Nectin-4, B7-H3, and B7-H4.
Pharmaceutical giants have also joined in the layout of the ADC pipeline. This year, Pfizer has acquired Seagen for as high as 43 billion US dollars, incorporating its ADC pipeline, including Nectin-4 ADC Padcev, CD30 ADC Adcetris, Zinc transporter LIV-1 ADC Ladiratuzumab vedotin, ITGB6 ADC SGN-B6A and B7H4 ADC SGN-B7H4V.
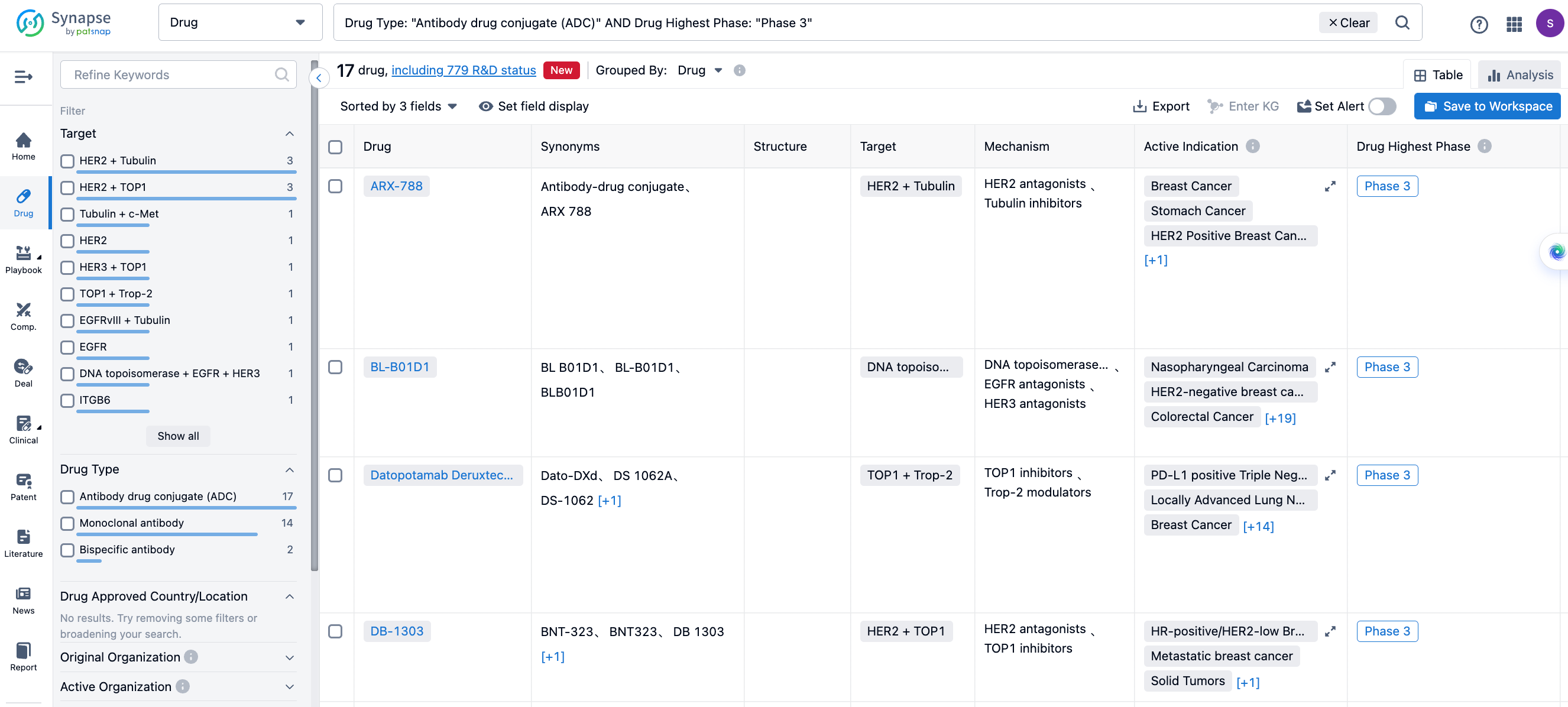
Global Phase III Clinical ADCs under Investigation
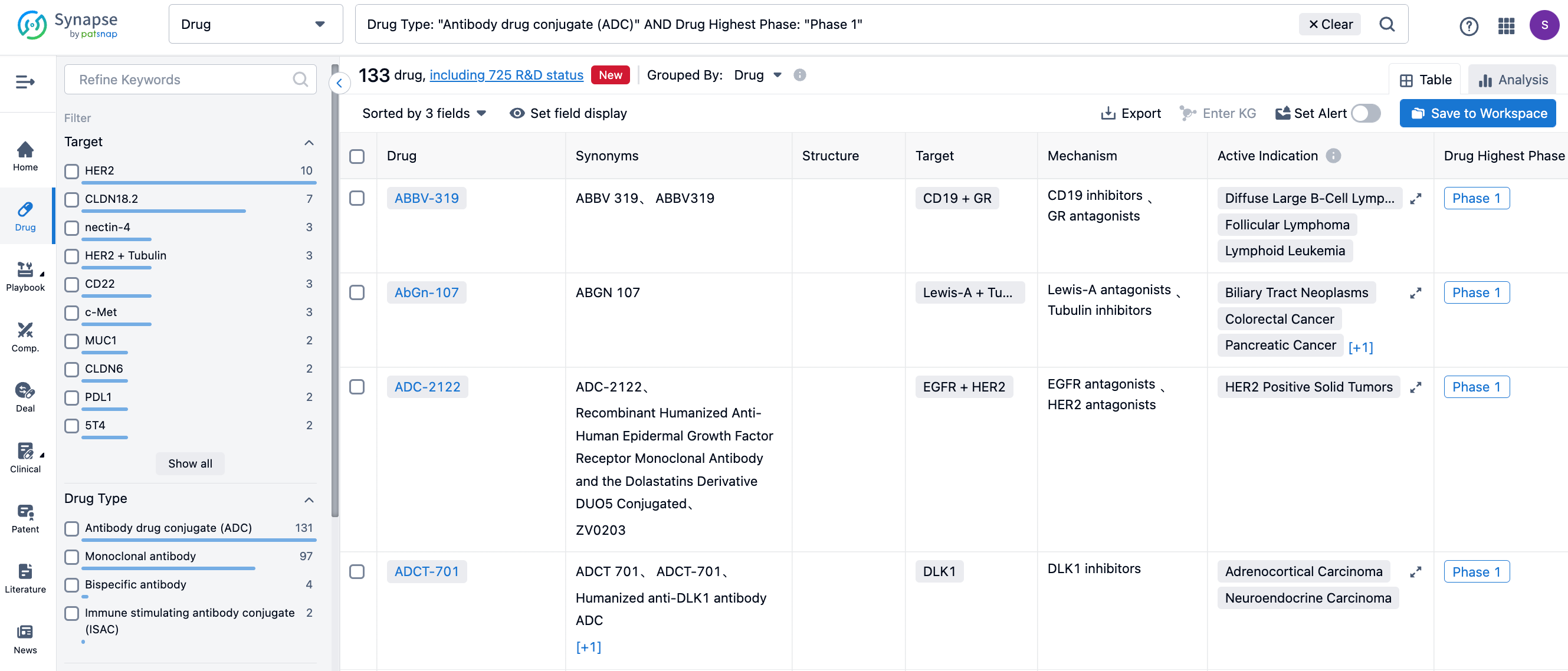
Apart from the approved ADCs, there are hundreds of ADCs under clinical investigation, covering a increasingly diverse range of targets. Not only do they include popular targets like HER2 and Trop-2, but also emerging targets such as B7-H3, FRα, Nectin-4, and so on.
There are several drugs globally that have entered phase III clinical stage. The figure below lists some of the ADCs under phase III clinical investigation.
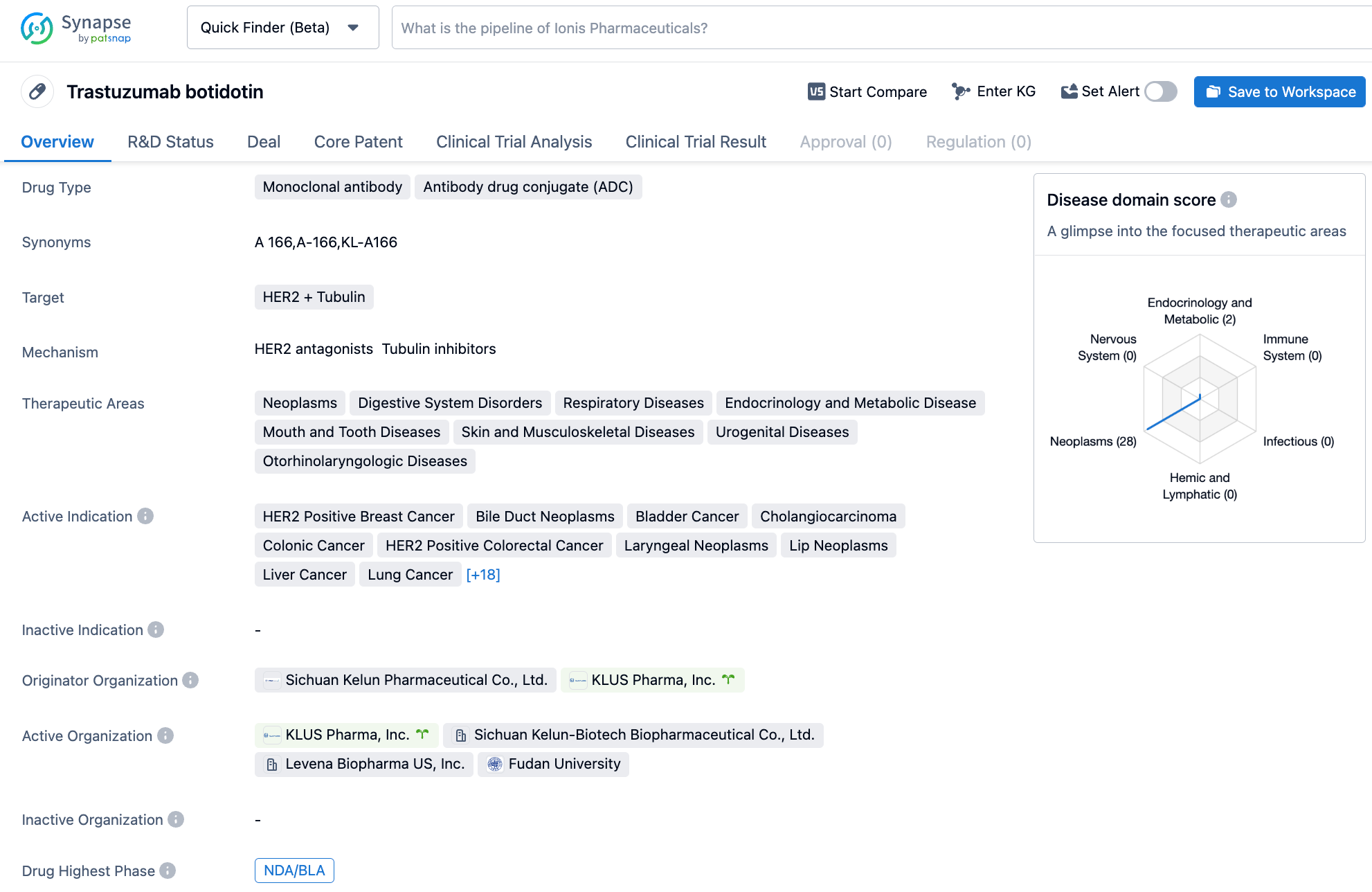
A166
A166 is a HER2 ADC developed by Sichuan Kelun-Biotech Biopharmaceutical, a subsidiary of Sichuan Kelun Pharmaceutical, using the OptiDC platform. By stably proteolytic cleavable valine-citrulline linker, it specifically links to the HER2 antibody site and couples it to Duo-5 (an antimicrotubule agent). It has demonstrated its safety and effectiveness in HER2-positive breast cancer patients.
Last year, at the ASCO conference, Sichuan Kelun-Biotech Biopharmaceutical announced the results of a phase I trial of A166 (CTR20181301). The best ORR among all patients in the 4.8 and 6.0 mg/kg groups was 73.91% and 68.57%, respectively, with mPFS of 12.30 and 9.40 months, respectively. Among 23 patients treated at a dose level of 4.8 mg / kg, one patient had confirmed and sustained complete remission for 7+ months[1].
The new drug application for A166 was accepted by the CDE on May 11th, for the treatment of HER2-positive locally advanced, inoperable, recurrent or metastatic breast cancer that has failed second-line and above anti-HER2 treatment.
Patritumab deruxtecan
Patritumab deruxtecan (HER3-DXd, U3-1402) is the world's first HER3-targeting antibody-drug conjugate (ADC) developed by Daiichi Sankyo, and is currently being investigated for indications such as breast cancer and non-small cell lung cancer (NSCLC).
HER3-DXd is created by conjugating the monoclonal antibody patritumab (U3-1287), which targets the extracellular domain of HER3, to the cytotoxic topoisomerase I inhibitor deruxtecan using a maleimidocaproyl-GGFG linker via a cysteine-specific coupling approach, with a drug-to-antibody ratio (DAR) of 8.
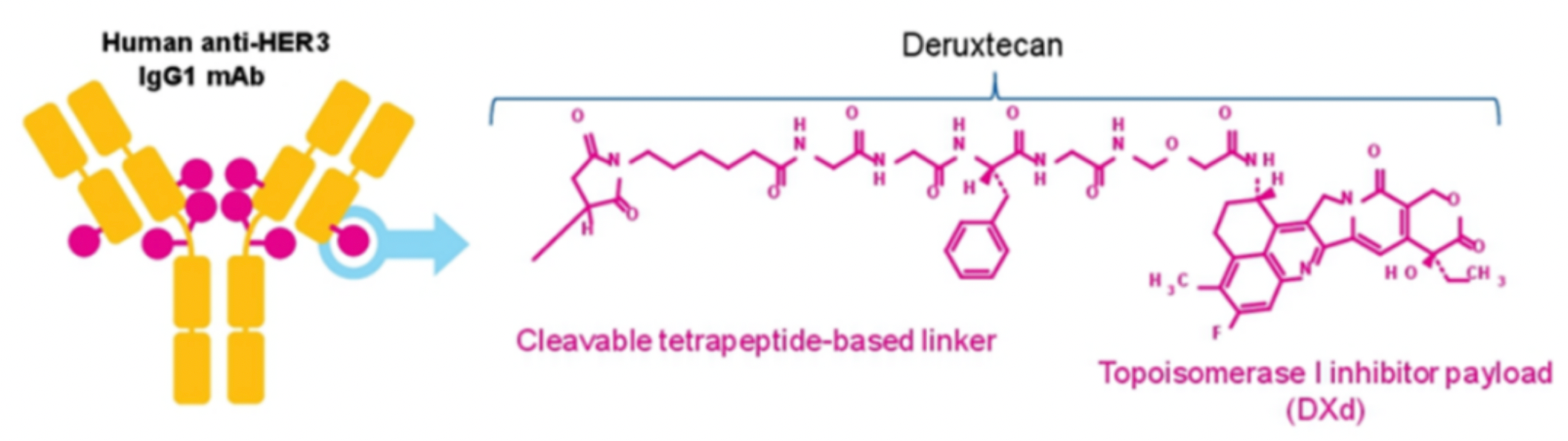
In an oral presentation at this year's ASCO meeting, Daiichi Sankyo unveiled Part A results of the Phase 2 study of HER3-Dxd in patients with HER2-negative metastatic breast cancer (MBC): the overall response rate (ORR) in the total population was 35%, the clinical benefit rate (CBR) was 48%, and the median duration of response (DOR) was 10.0 months, with a 6-month progression-free survival (PFS) rate of 60%.
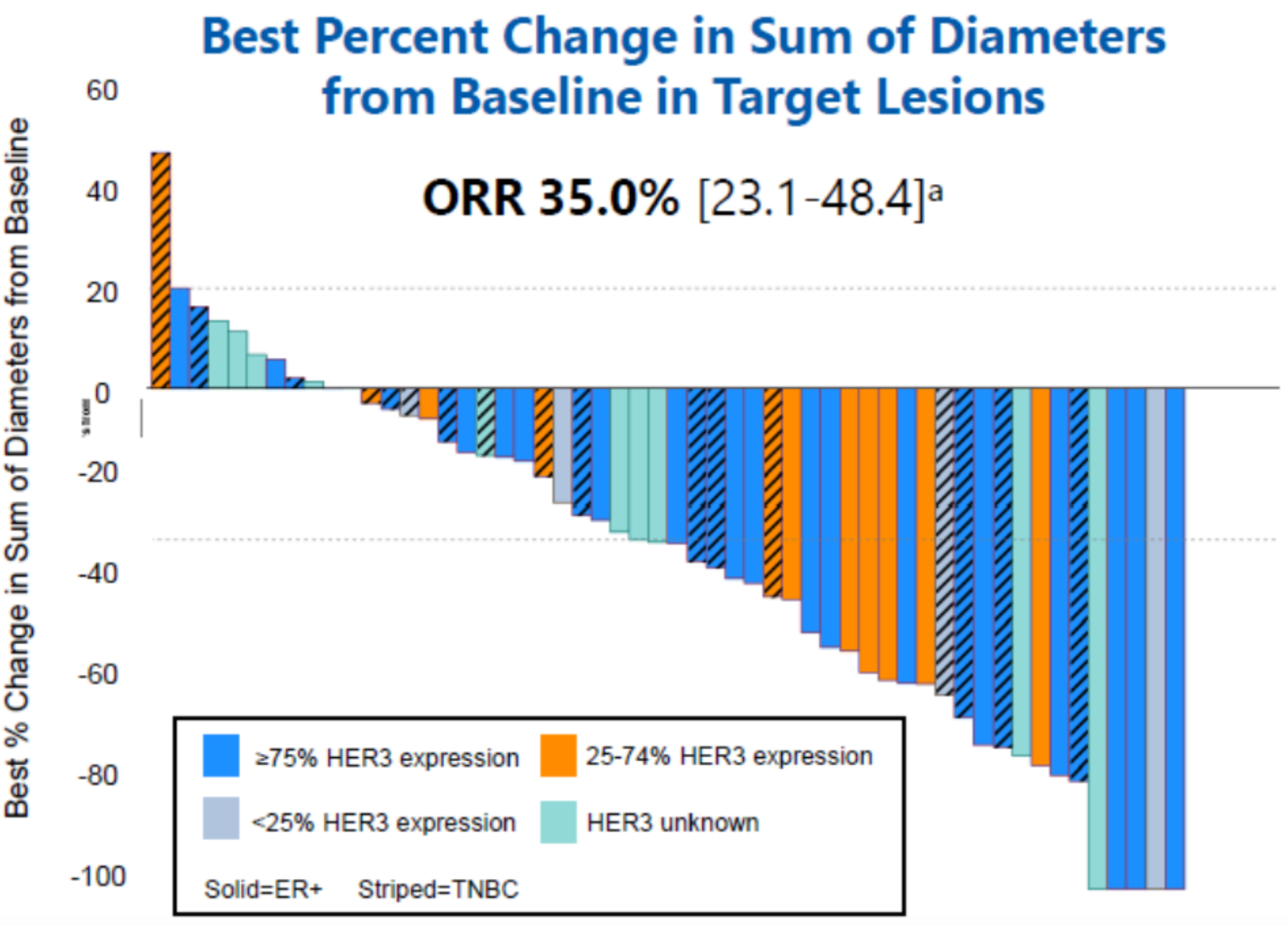
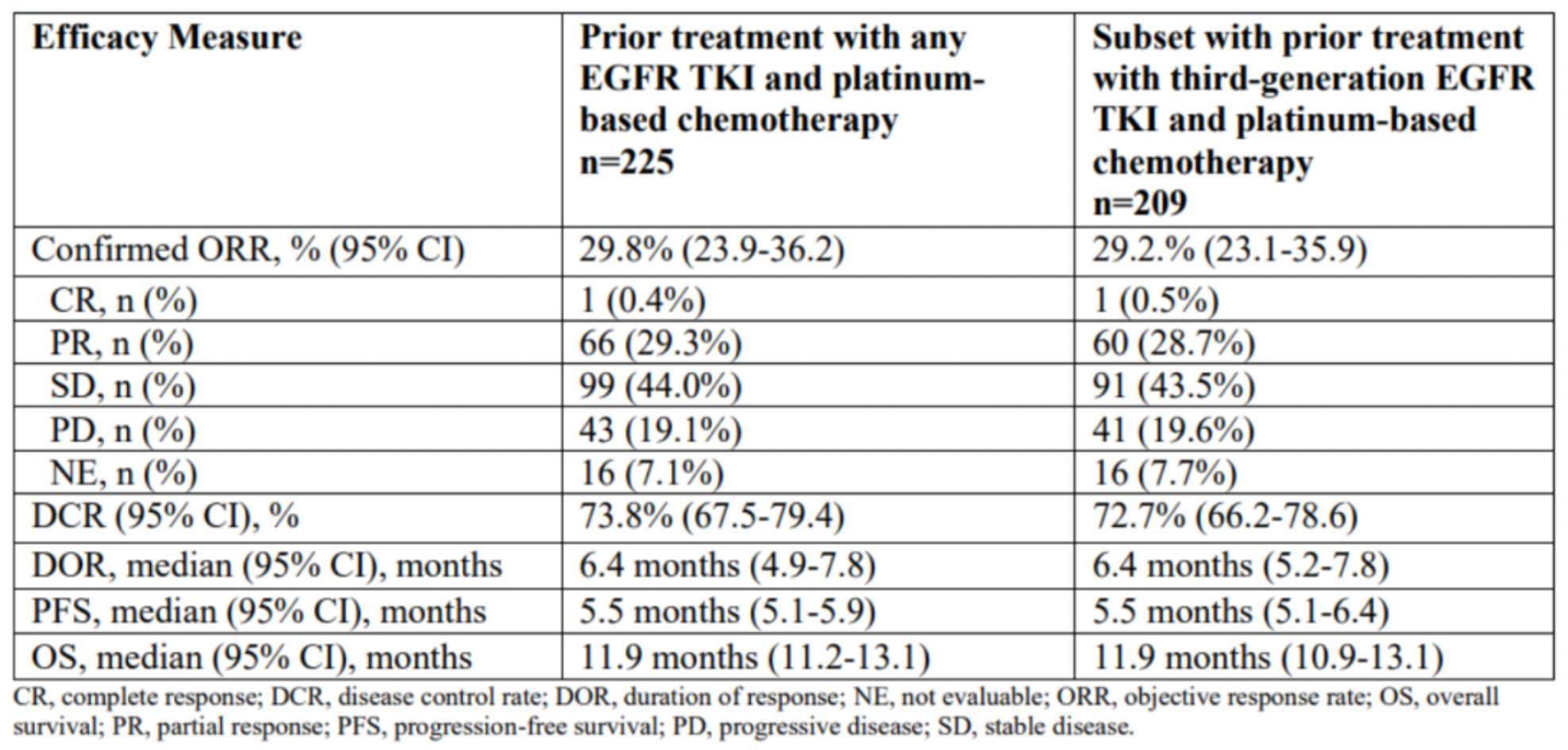
More recently, at the 2023 WCLC meeting, Daiichi Sankyo announced the key results of the HERTHENA-Lung01, a pivotal Phase 2 study of HER3-DXd in patients with EGFR-mutated non-small cell lung cancer. Evaluated by blinded independent central review (BICR), in 225 patients with EGFR mutation-positive NSCLC treated with HER3-DXd (5.6mg/kg), a confirmed ORR of 29.8% was observed, including 1 complete response (CR), 66 partial responses (PR), and 99 cases of stable disease (SD). The DOR was 6.4 months, with a disease control rate (DCR) of 73.8%. As of May 18, 2023, the median progression-free survival (PFS) was 5.5 months, and the median overall survival (OS) was 11.9 months.
Moreover, Merck (known as MSD outside of the United States and Canada) has recently acquired three ADCs, including HER3-Dxd, with a total purchase price of $22 billion, indicating Merck's significant optimism for the potential of this drug.
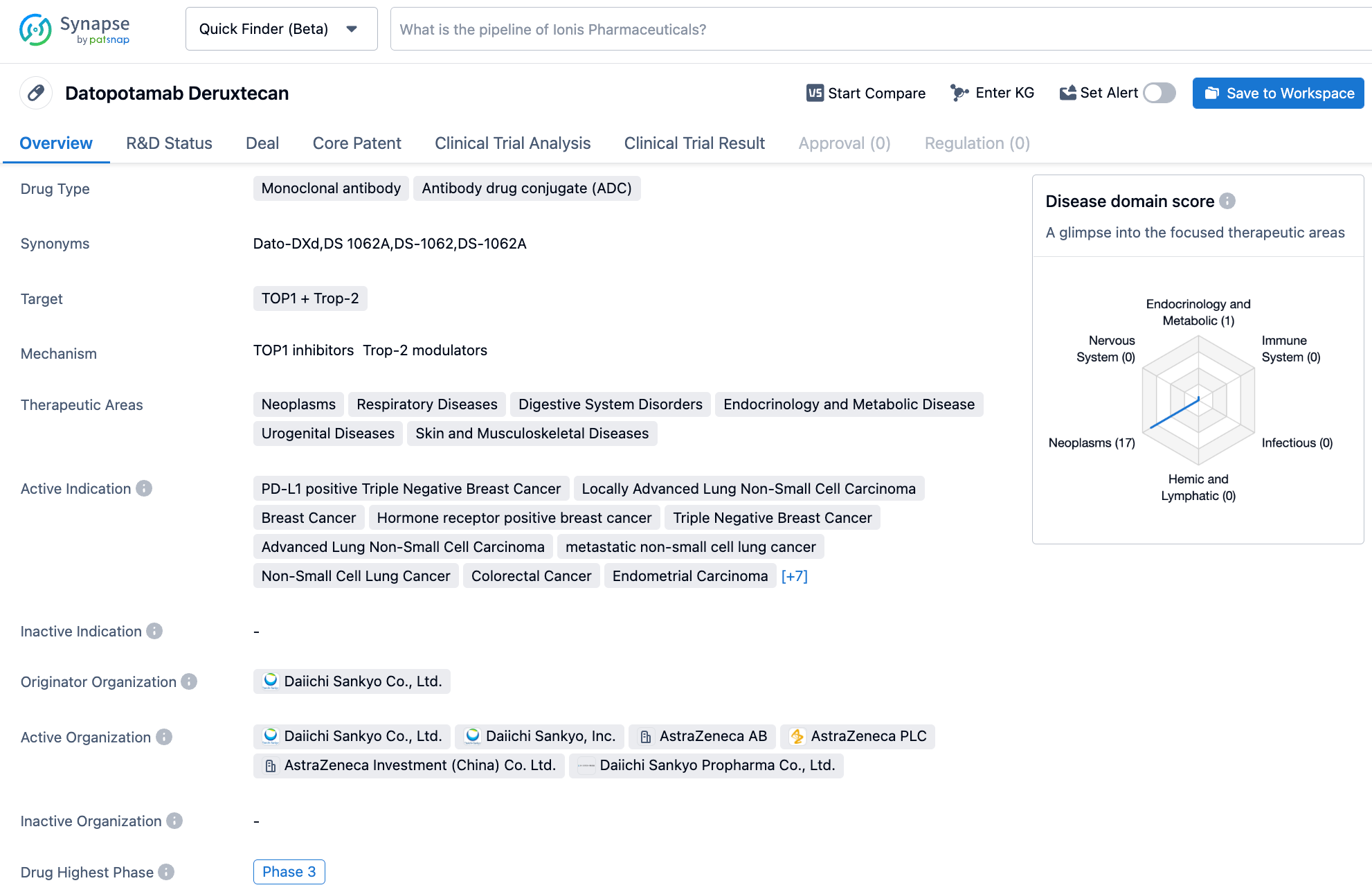
Datopotamab deruxtecan
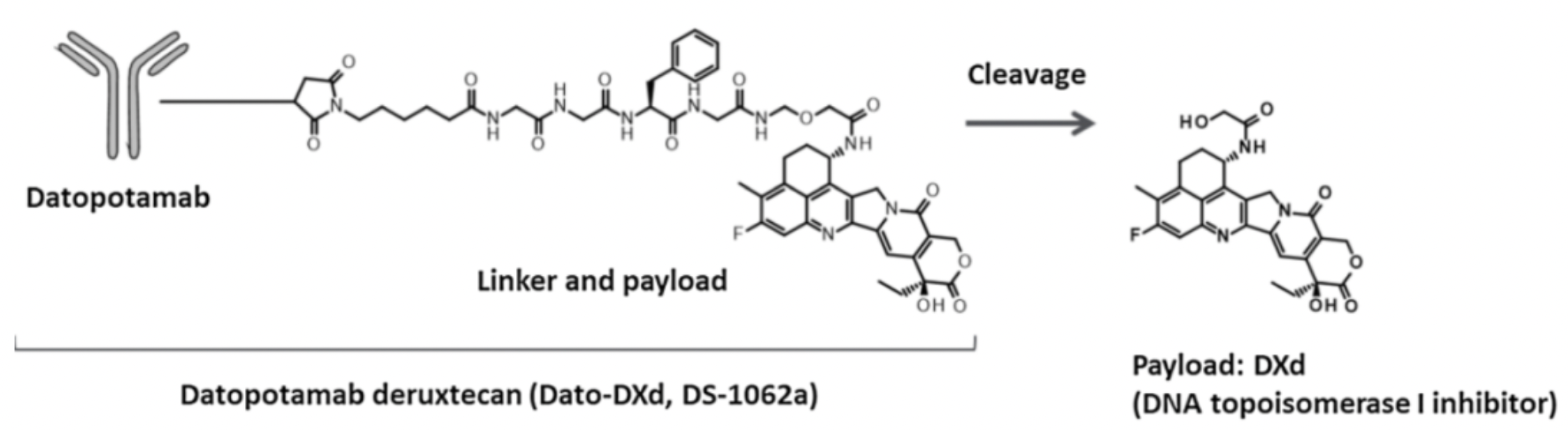
Datopotamab deruxtecan (Dato-DXd, DS-1062) is a Trop-2-targeted antibody-drug conjugate (ADC) co-developed by AstraZeneca and Daiichi Sankyo. It is constructed by coupling a recombinant humanized anti-Trop-2 IgG1 antibody to a topoisomerase I inhibitor (DXd) via a tetrapeptide-based cleavable linker designed to reduce cysteine residues on the interchain disulfide bonds of datopotamab. The tetrapeptide-based linker is designed to release DXd following proteolytic processing by lysosomal enzymes, such as cathepsin, upon internalization into cancer cells. On average, the goal is to have an average of four drug-to-antibody ratio (DAR) per antibody molecule.
Unlike Trodelvy, the first approved Trop-2 ADC for the treatment of breast cancer, AstraZeneca and Daiichi Sankyo plan to focus on advanced non-small cell lung cancer (NSCLC) as the initial indication for Dato-DXd, positioning it as a potential alternative to chemotherapy in the treatment of NSCLC.
At this year's ASCO Annual Meeting, AstraZeneca and Daiichi Sankyo presented study results for Dato-DXd in the treatment of advanced NSCLC from the TROPION-Lung02 study: Dato-DXd combined with durvalumab or Dato-DXd combined with durvalumab plus platinum-based chemotherapy resulted in an objective response rate (ORR) of 38% and 49%, disease control rates (DCR) of 84% and 87%, and progression-free survival (PFS) of 8.3 months and 7.3 months, respectively, in the overall advanced NSCLC population. In the first-line treatment population, the ORR for Dato-DXd combined with durvalumab or with durvalumab plus platinum-based chemotherapy was 50% and 57%, respectively, with a DCR of 91% for both combinations, and the median duration of response (DOR) had not yet been reached. These results further confirm the significant efficacy and good safety profile of Dato-DXd combined with durvalumab ± platinum-based chemotherapy in first-line or previously treated advanced NSCLC patients without actionable genomic alterations.
However, Dato-DXd has also encountered safety issues. Although the TROPION-Lung01 trial met its primary clinical endpoint, serious safety concerns arose, including cases of interstitial lung disease and Grade 5 adverse events leading to death due to drug-related adverse reactions. This news had a significant impact on both AstraZeneca and Daiichi Sankyo, causing their stock prices to drop by 8% and 15% respectively on the day of the announcement.
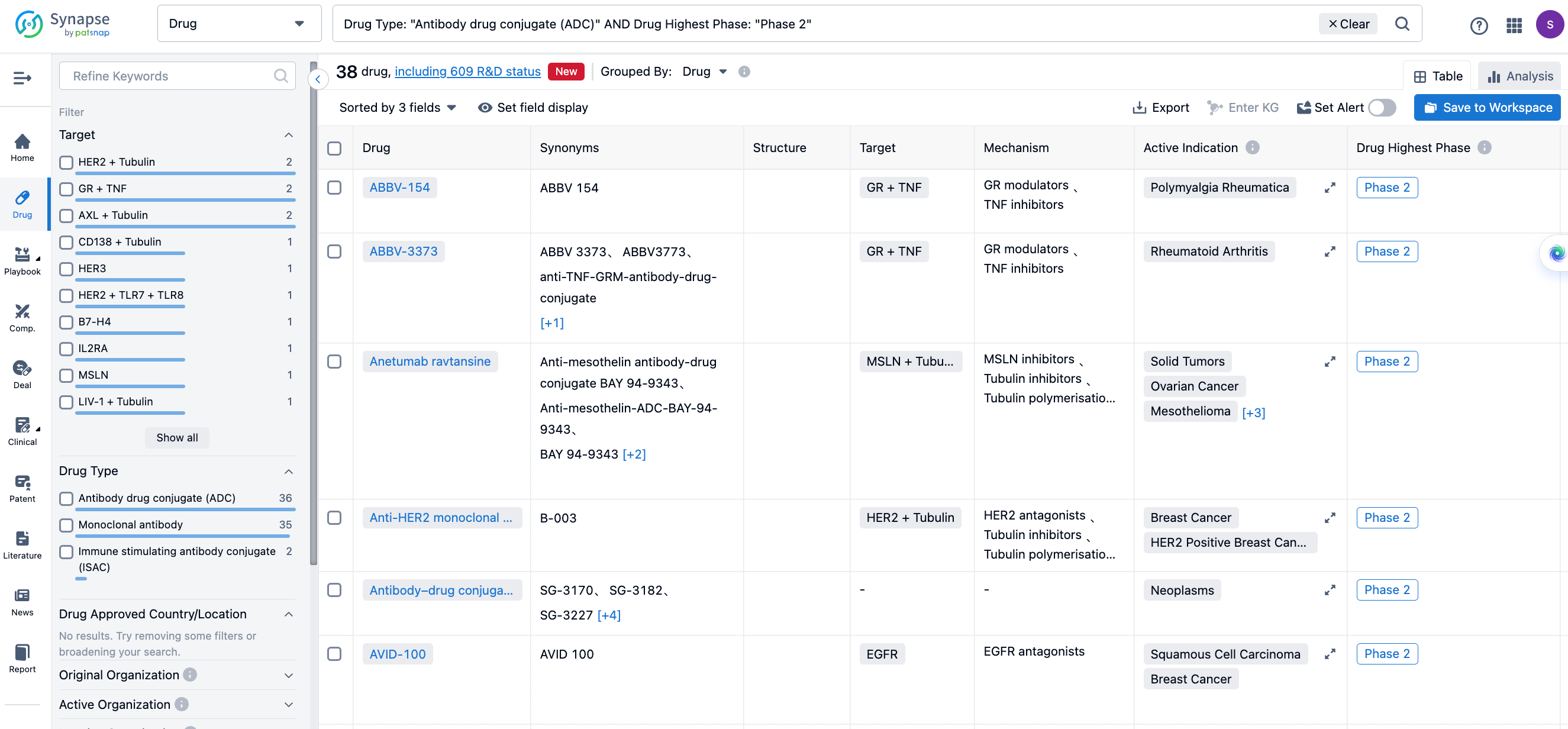
Global Phase II Clinical ADCs under Investigation
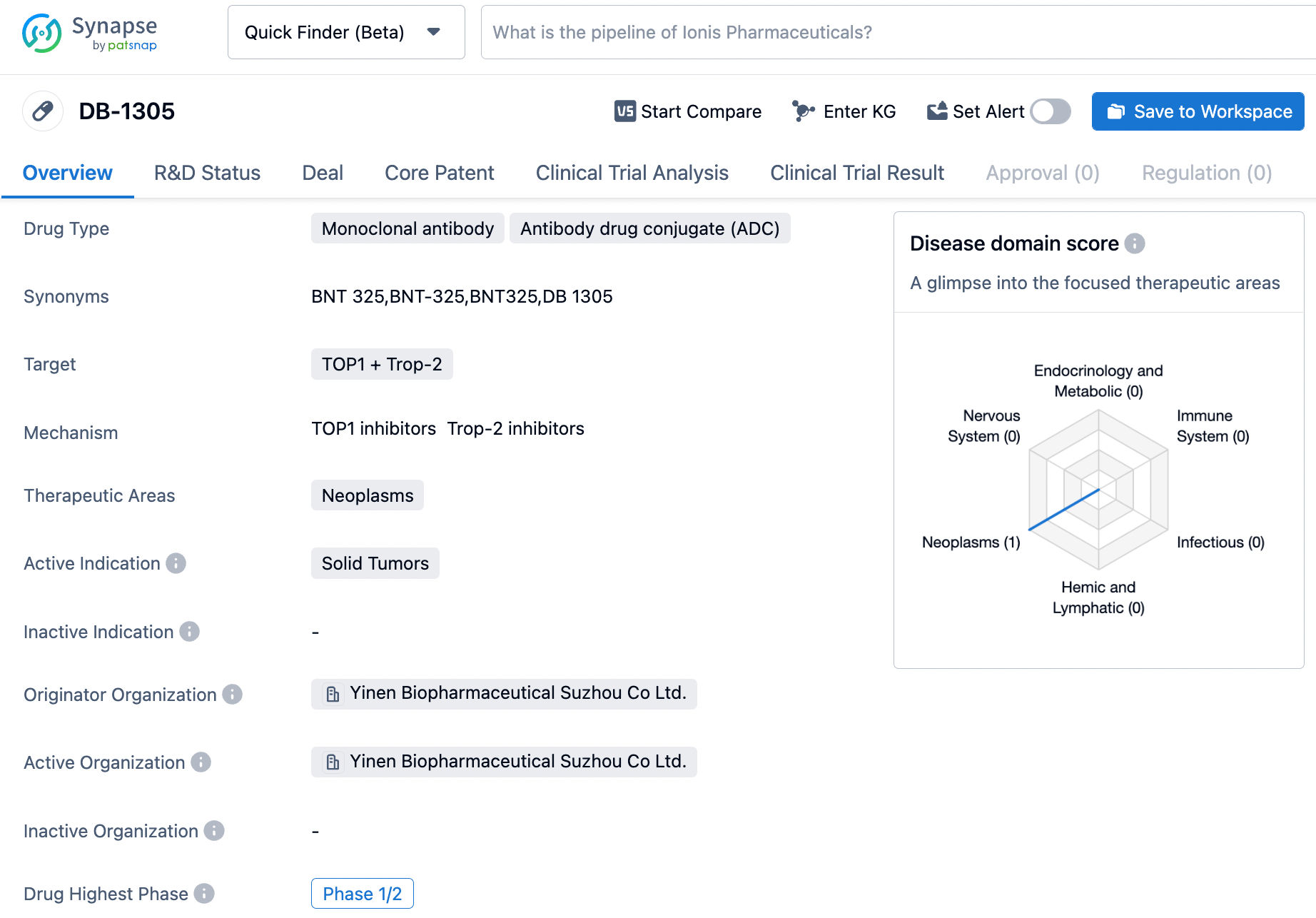
DB-1305
DB-1305 is a Trop-2 targeted ADC developed by Yingen Biopharmaceutical based on its proprietary Duality ImmunoToxin Antibody Conjugate (DITAC) platform, coupling a Trop-2 antibody to an innovative topoisomerase I inhibitor, P1021, through an enzymatically cleavable tetrapeptide linker.
In June 2022, DB-1305 initiated an open-label, multi-center, multi-dose, phase 1/2a study (NCT05438329), which included a dose-escalation phase 1 in pretreated patients with advanced solid tumors and a dose-expansion phase 2a. As of December 19, 2022, 20 patients have participated in the dose-escalation portion of the study.
Currently, Yingen Biopharmaceutical is co-developing, producing, and commercializing DB-1305 in partnership with BioNTech. This collaboration follows BioNTech's acquisition of two ADC products from Yingen Biopharmaceutical for $1.67 billion in April 2023, making DB-1305 the third product the two companies are jointly advancing.
9MW2821
9MW2821 is an ADC (Antibody-Drug Conjugate) targeting Nectin-4, developed by Jiangsu Maiweikang New Drug Research and Development Co. It received domestic clinical approval in China in October 2021, with indications including urothelial carcinoma, breast cancer, lung cancer, and several other solid tumors.
9MW2821 is produced by employing inter-chain disulfide drug conjugation technology to link a humanized antibody with the cytotoxic payload monomethyl auristatin E (MMAE).
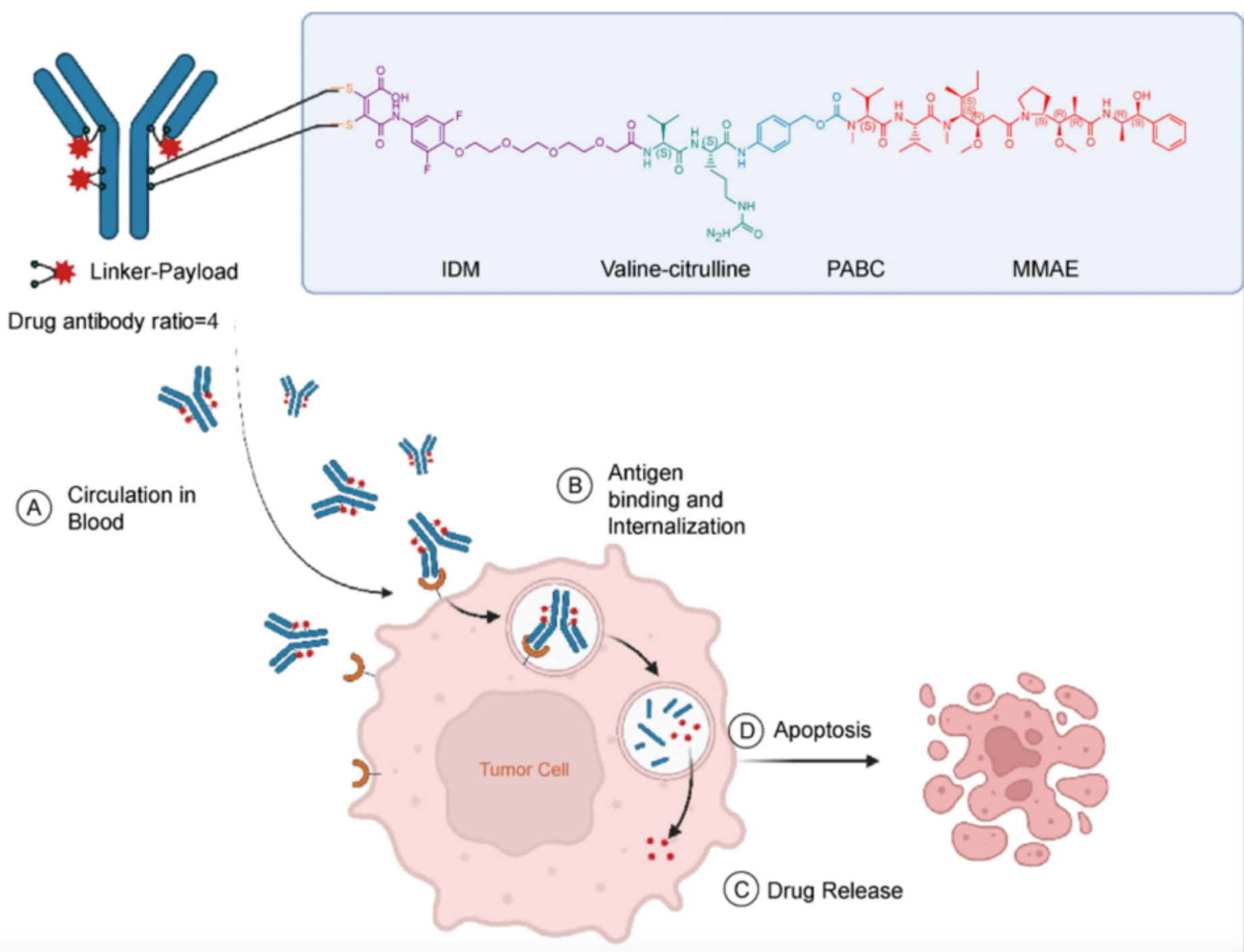
The ADC 9MW2821 is currently undergoing Phase 1/2 clinical trials (NCT05216965 and NCT05773937) in patients with advanced solid tumors, aiming to evaluate the safety, tolerability, pharmacokinetics, preliminary antitumor activity, and immunogenicity of 9MW2821 administered intravenously.
Global Phase I Clinical ADCs under Investigation
Mirzotamab clezutoclax
Mirzotamab clezutoclax (ABBV-155; Mirzo-C) is a B7-H3-targeted Antibody-Drug Conjugate (ADC) developed by AbbVie, comprising three components: an anti-B7-H3 monoclonal antibody, a cleavable linker, and a BCL-XL inhibitor payload. Specific targeting of BCL-XL has demonstrated synergy with other anticancer drugs in preclinical models.
At this year's ASCO conference, AbbVie released the results of a Phase 1 open-label study (NCT03595059) for ABBV-155, evaluating the efficacy of ABBV-155 in combination with taxane-based chemotherapy.
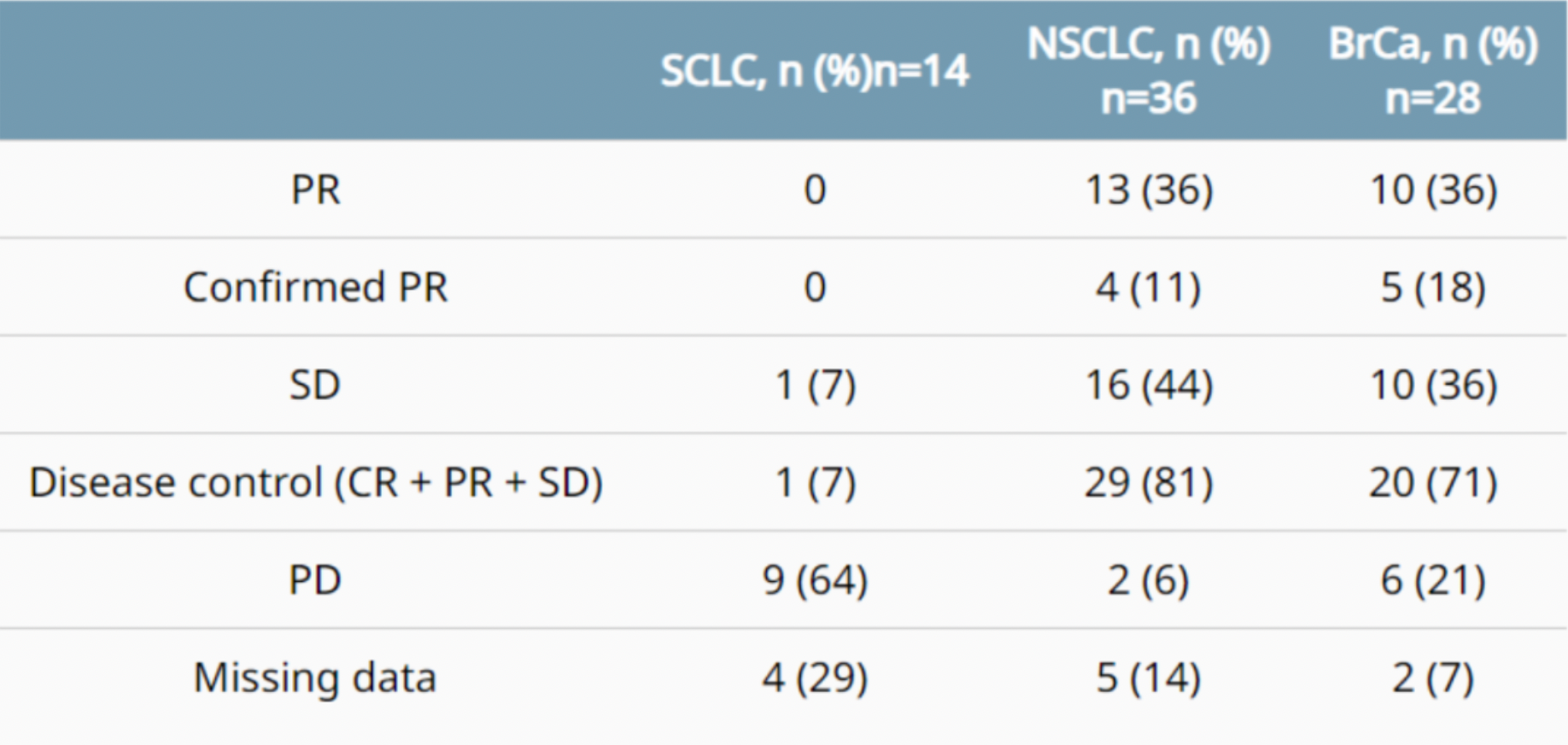
As of November 18, 2022, a total of 78 patients had been enrolled (small cell lung cancer n=14; non-small cell lung cancer n=36; breast cancer n=28).
In terms of efficacy, the confirmed objective response rate (cORR) for the SCLC cohort was 0%, with a 0% partial response (PR) rate; the NSCLC cohort had a cORR of 11% and a PR rate of 36%; the breast cancer cohort exhibited a cORR of 18% and PR rate of 36%. Overall, ABBV-155, whether used as a monotherapy or in combination with docetaxel, displayed favorable tolerability.
SGN-B6A
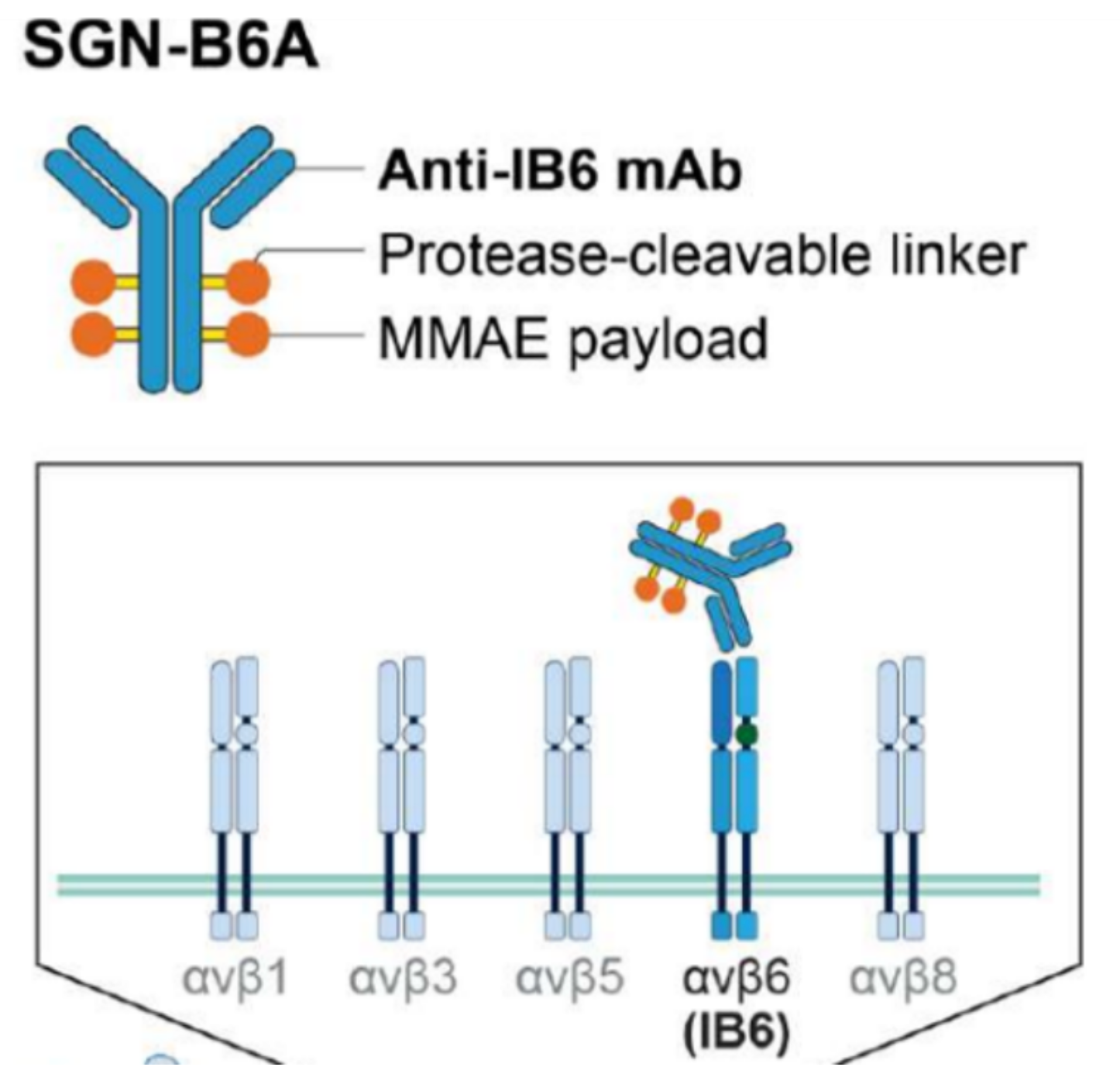
SGN-B6A is an antibody-drug conjugate (ADC) developed by Seagen, targeting integrin β-6 (ITGB6) to deliver the clinically validated potent payload monomethyl auristatin E (MMAE) to cancer cells. The antibody component of SGN-B6A is specific for integrin β-6 and does not bind to other members of the α-v family. SGN-B6A mediates anti-tumor activity through MMAE-induced cytotoxicity, bystander effects, and immunogenic cell death.
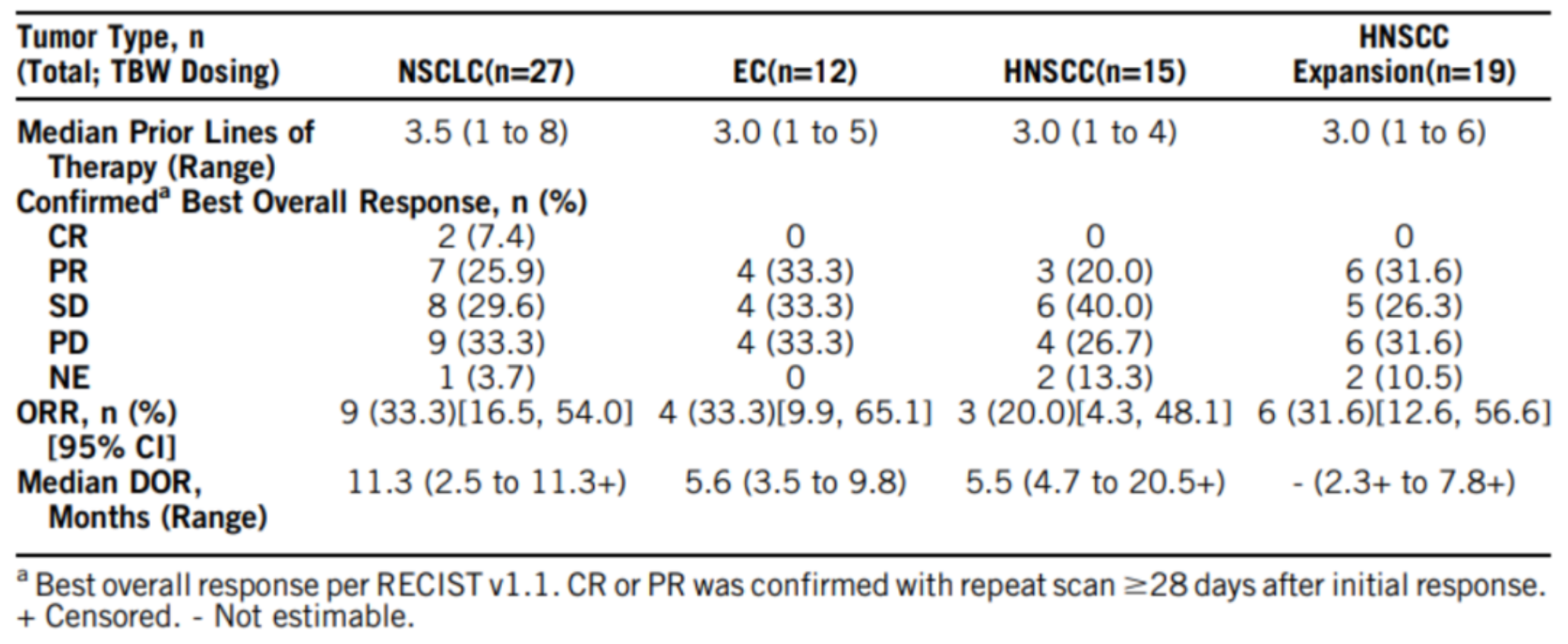
At this year's ASCO meeting, Antoine and colleagues presented the results of a phase 1 clinical trial (NCT04389632) for SGN-B6A. As of December 14, 2022, 148 patients were treated with SGN-B6A, including 88 patients in part A and 60 patients in part B of the study, with enrollment for part A completed. SGN-B6A demonstrated a manageable safety profile and showed encouraging preliminary anti-tumor activity with dose-escalated durability of response in a heavily pretreated population. Dose-expansion cohorts of part B for non-small cell lung cancer (NSCLC), esophageal squamous cell carcinoma (ESCC), and head and neck squamous cell carcinoma (HNSCC) are currently ongoing.
Numerous antibody-drug conjugate (ADC) therapies have been successfully developed to date, benefiting tens of thousands of cancer patients. As of now, there are 15 ADCs that have been globally approved for marketing. The market presence of these ADCs, along with the impressive clinical performance of various ADCs, has garnered increased attention in this field.
In addition, there are several hundred ADCs currently under research around the world, targeting markers such as HER2, Trop-2, B7-H3, c-Met, and EGFR, among others.
This article summarizes a portion of the ADCs in clinical research and briefly introduces some of the typical compounds among them, with the belief that more ADCs will be approved and reach the market in the near future.