Carisma Therapeutics Reveals Regulatory Green Light for Experimental HER2-Focused CAR-Monocyte Therapy CT-0525 by FDA
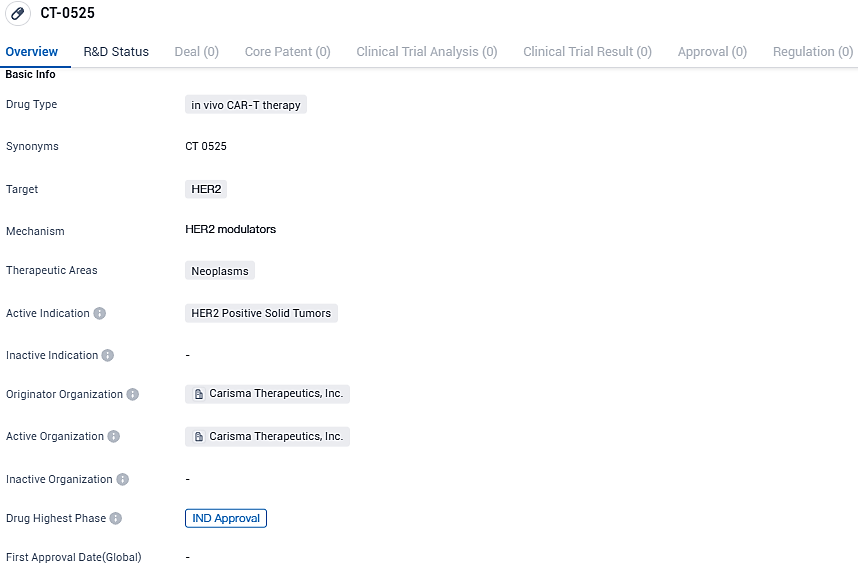
Carisma Therapeutics Inc., an enterprise at the clinical research phase dedicated to pioneering new immune-based treatments, has confirmed that the U.S. Food and Drug Administration (FDA) has approved its Investigational New Drug (IND) application for their novel product, CT-0525. This groundbreaking therapy utilizes genetically altered autologous CAR-monocytes designed to target and combat solid cancers that have elevated levels of the human epidermal growth factor receptor 2 (HER2).
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
After obtaining authorization from the FDA to go ahead with their investigation, Carisma anticipates launching a Phase 1 trial shortly and plans to administer treatment to the inaugural participant by mid-2024.
"Receiving the IND approval for CT-0525 marks a crucial advancement in Carisma's pursuit of pioneering therapies targeting myeloid cells for patients with metastatic solid tumors," commented Steven Kelly, the CEO and President of Carisma. "Our objectives for the Phase 1 trial include evaluating CT-0525's safety profile, its tolerability, the feasibility of its production, and exploring how it functions."
Monocytes, the progenitors of macrophages, could offer several advantages in the CAR-Monocyte therapeutic strategy to address particular challenges in solid tumor treatments. The CAR-Monocyte production platform boasts the capacity to generate up to 10 billion cells following a single apheresis session, featuring an expedited one-day production cycle.
This innovative cell production process may lead to substantial reductions in the cost and production times related to these personally tailored cellular therapies. At the Society for Immunotherapy of Cancer's most recent annual meeting, held in November 2022, Carisma unveiled preliminary results indicating that CT-0525 lessened the progression of tumorous growth in a number of solid tumor models examined before clinical trials.
"CT-0525 represents the pioneering evaluation of a CAR-Monocyte in the context of solid tumors. It exhibits prolonged activity once in the body, can evolve into pro-inflammatory CAR macrophages, and attacks tumors through multiple mechanisms. In addition, with its ability to produce an abundant number of cells, CT-0525 could potentially revolutionize how we treat patients with metastatic solid tumors that have an excess of HER2," explained Michael Klichinsky, PharmD, PhD, Co-Founder and CSO of Carisma.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of December 7, 2023, there are 530 investigational drugs for the HER2 target, including 175 indications, 554 R&D institutions involved, with related clinical trials reaching 3396, and as many as 53315 patents.
CT-0525 is an ex vivo gene-modified autologous chimeric antigen receptor-monocyte cellular therapy intended to treat solid tumors that overexpress human epidermal growth factor receptor 2. The CAR-Monocyte approach has the potential to address the challenges of treating solid tumors with cell therapies, including tumor infiltration, immunosuppression within the tumor microenvironment, and antigen heterogeneity.