Celldex Initiates Global Phase 3 Trial of Barzolvolimab for Chronic Spontaneous Urticaria
Celldex Therapeutics, Inc. has commenced its global Phase 3 program, comprising two Phase 3 clinical studies aimed at determining the efficacy and safety of barzolvolimab in adult patients with CSU who continue to exhibit symptoms despite undergoing H1 antihistamine treatment. These trials will additionally include patients who remain symptomatic following treatment with biologics.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.

For patients dealing with Chronic Spontaneous Urticaria (CSU), the activation of mast cells in the skin triggers episodes of itchy hives, swelling, and inflammation, which can greatly disrupt their everyday lives for extended periods, ranging from years to decades. Treatment options are quite limited, especially for those who do not respond adequately to omalizumab. Barzolvolimab, an innovative monoclonal antibody, operates upstream of other CSU treatment methods, addressing the root cause of the condition by obstructing the receptor tyrosine kinase KIT, indispensable for mast cell operation and survival.
“Barzolvolimab has shown an exceptional and profound capacity to provide swift, lasting, and complete disease control for CSU patients, regardless of previous treatment histories, across multiple studies,” said Marcus Maurer, MD, Professor of Dermatology and Allergy at Charité – Universitätsmedizin Berlin, and a Principal Investigator in the Phase 3 barzolvolimab studies.
Maurer emphasized that “progressing a promising compound that confronts the root cause—mast cell dysfunction—into late-stage trials is a significant and encouraging step for both patients and physicians who urgently require more effective treatment options.”
Anthony Marucci, Co-founder, President, and CEO of Celldex Therapeutics, added, “There is a critical need for treatments that deliver complete disease control for more CSU patients. Advancing barzolvolimab into pivotal registrational studies marks an important step forward for these patients. We are committed to conducting these trials efficiently and aim to make barzolvolimab available to meet the needs of CSU patients, while also extending its application to additional indications.”
In a placebo-controlled Phase 2 study for CSU, barzolvolimab achieved the primary endpoint, showing a statistically significant mean change from baseline to week 12 in UAS7 across all dosages. Secondary and exploratory endpoints were also met at week 12, robustly supporting the primary results, including changes in ISS7 and HSS7, and responder analyses.
Notably, barzolvolimab produced rapid, lasting, and clinically significant responses in patients with moderate to severe CSU unresponsive to antihistamines, including those with prior omalizumab treatment. It was well tolerated, showcasing an encouraging safety profile.
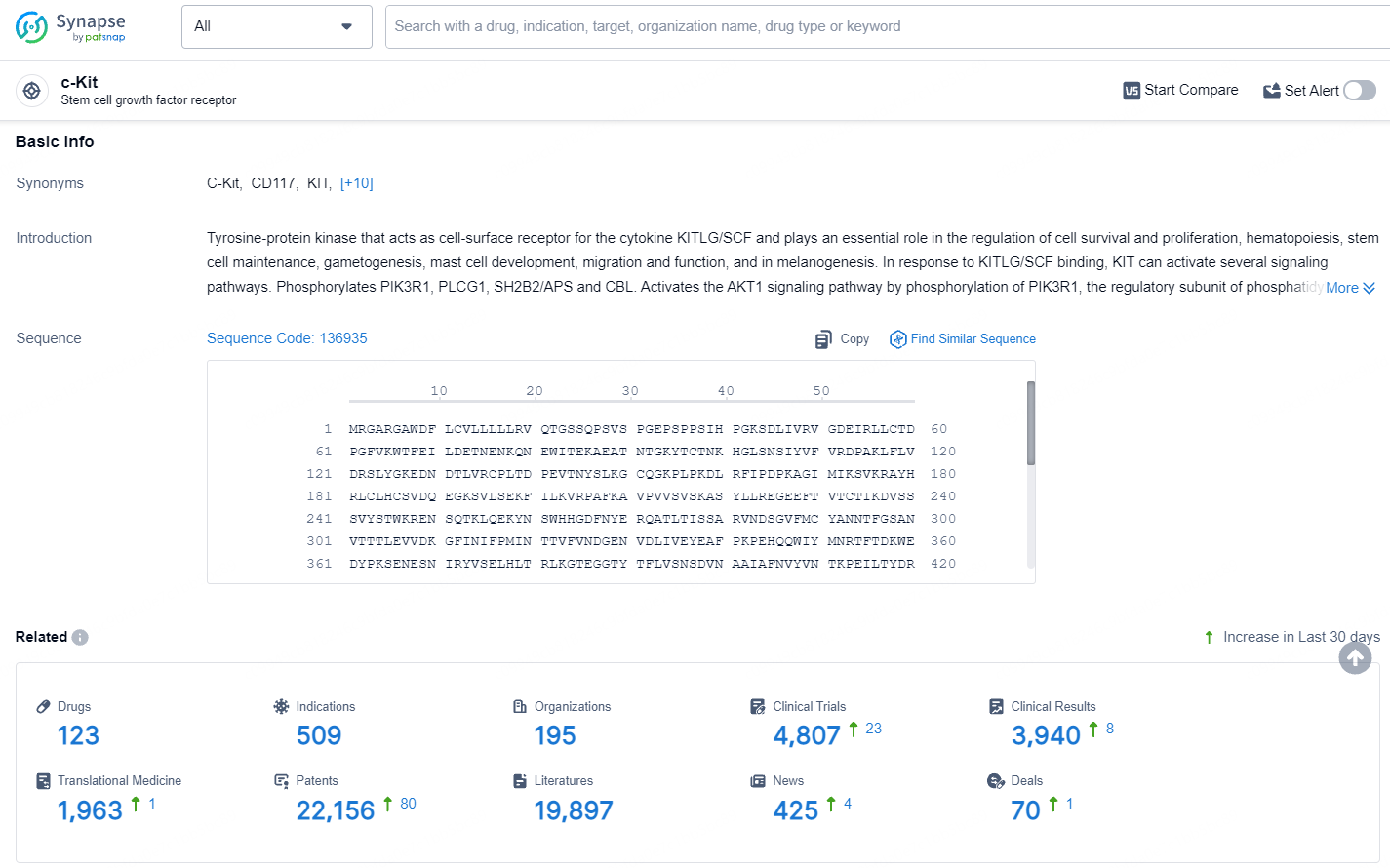
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of July 22, 2024, there are 123 investigational drugs for the c-Kit target, including 509 indications, 195 R&D institutions involved, with related clinical trials reaching 4807, and as many as 22156 patents.
Barzolvolimab as a monoclonal antibody targeting c-Kit represents a significant advancement in the potential treatment of immune system diseases, digestive system disorders, hemic and lymphatic diseases, and skin and musculoskeletal diseases. As the drug progresses through the later stages of clinical development, its impact on patient outcomes and its potential role in clinical practice will become clearer.