Celldex Initiates Phase 1 Trial for CDX-622 in Healthy Volunteers
Celldex Therapeutics, Inc. (NASDAQ:CLDX) has reported that dosing has commenced for the first participant in its Phase 1a clinical trial of CDX-622 involving healthy volunteers. CDX-622 functions as a bispecific antibody, addressing two synergistic pathways that promote chronic inflammation. It effectively neutralizes the alarmin thymic stromal lymphopoietin (TSLP) and reduces mast cell populations by depleting them through stem cell factor (SCF) deprivation.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
"The launch of our initial bispecific candidate for inflammatory conditions, CDX-622, underscores our expertise in mast cell biology," stated Anthony Marucci, Co-founder, President, and CEO of Celldex Therapeutics. "CDX-622 integrates mast cell depletion with the suppression of Type 2 inflammatory pathways, potentially allowing for extensive use across various respiratory and dermatological conditions. Following the successful conclusion of this trial involving healthy subjects, we are eager to establish a strong pipeline, starting with a study focused on asthma. Notably, we believe that CDX-622 will enhance our barzolvolimab program, bolstering our current pipeline that is advancing in five distinct diseases."
TSLP is directly linked with numerous respiratory and skin disorders, including asthma, chronic obstructive pulmonary disease, eosinophilic esophagitis, atopic dermatitis, and chronic spontaneous urticaria, as well as fibrotic conditions like systemic sclerosis and idiopathic pulmonary fibrosis. In these diseases, TSLP often shows increased expression correlating with the severity of conditions. Likewise, mast cells play a crucial role in the pathophysiology of allergic, inflammatory, autoimmune, and fibrotic diseases. CDX-622 features a distinctive SCF neutralizing ability that is anticipated to inhibit and deplete mast cells. The combined neutralization of SCF and TSLP via CDX-622 is expected to effectively lower tissue mast cells and suppress Type 2 inflammatory responses, potentially providing greater therapeutic advantages for inflammatory and fibrotic diseases.
The Phase 1a clinical trial comprises a two-part, randomized, double-blind, placebo-controlled, dose escalation study aimed at evaluating the safety, pharmacokinetics, and pharmacodynamics of ascending single doses (Part 1) and multiple doses (Part 2) of CDX-622 among up to 56 healthy participants. In Part 1, a single injection of either CDX-622 or placebo will be administered intravenously. For Part 2, CDX-622 or placebo will be given every 3 weeks for a total duration of up to 6 weeks post the initial dose, culminating in 3 doses. Participants will be monitored for 12 weeks across both Parts 1 and 2 after receiving their last dose of the study medication. Celldex will also investigate blood and skin biomarkers linked with SCF and TSLP signaling, as well as other immune inflammatory pathways in healthy subjects as exploratory endpoints. A subcutaneous formulation is currently under development and is set to be incorporated into this study in 2025.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
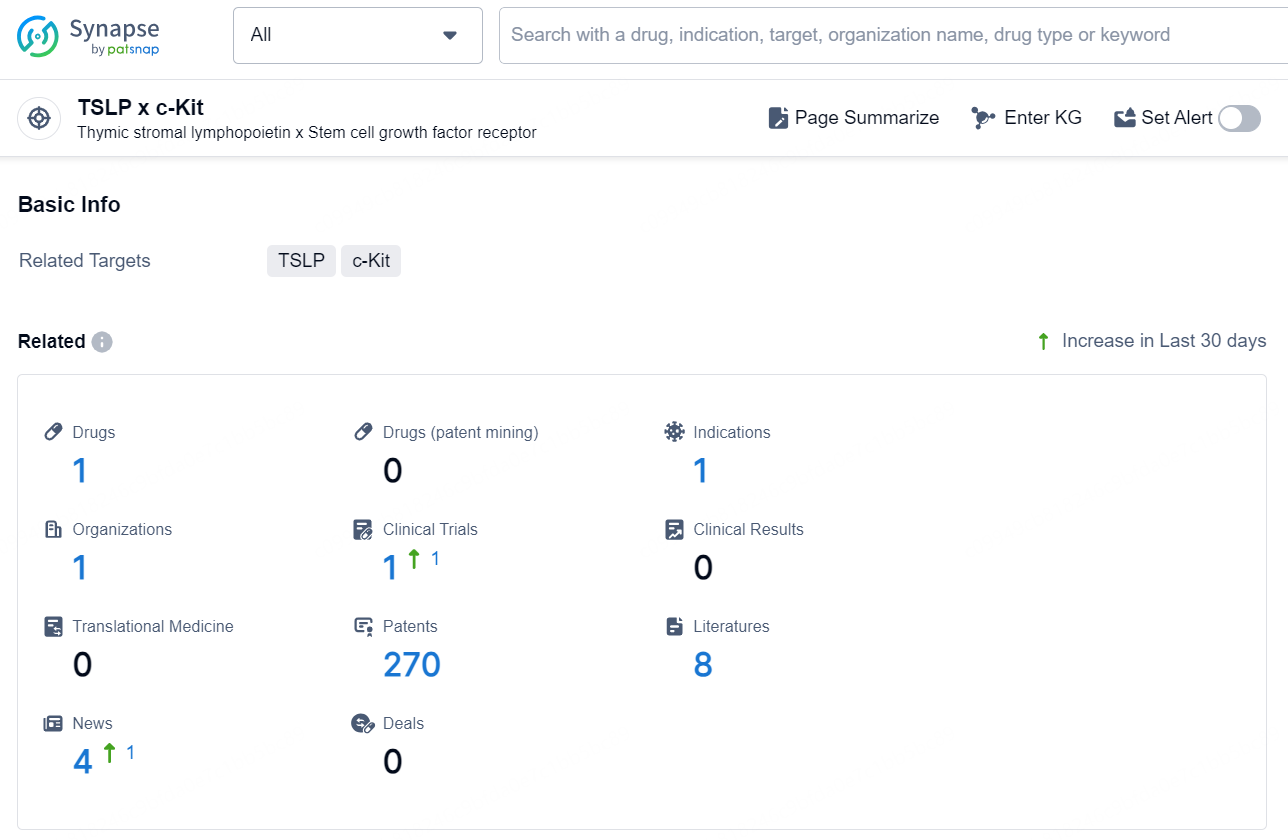
According to the data provided by the Synapse Database, As of November 26, 2024, there are 1 investigational drug for the TSLP x c-Kit target, including 1 indication, 1 R&D institution involved, with related clinical trial reaching 1, and as many as 270 patents.
CDX-622 is a bispecific antibody drug developed by Celldex Therapeutics, Inc. The drug targets Thymic Stromal Lymphopoietin (TSLP) and c-Kit and is intended for use in the treatment of inflammation in therapeutic areas other than diseases. As of the latest available data, CDX-622 is in the highest phase of development, which is Phase 1.