Checkpoint Therapeutics Resubmits Cosibelimab Biologics License Application
Checkpoint Therapeutics, Inc., an immunotherapy and targeted oncology firm in the clinical-stage, has announced the successful resubmission of its Biologics License Application (BLA) to the U.S. Food and Drug Administration (FDA) for cosibelimab. cosibelimab is their anti-programmed death ligand-1 (“PD-L1”) antibody, proposed as a new therapeutic option for individuals with metastatic or locally advanced cutaneous squamous cell carcinoma who are not suitable for curative surgery or curative radiation.
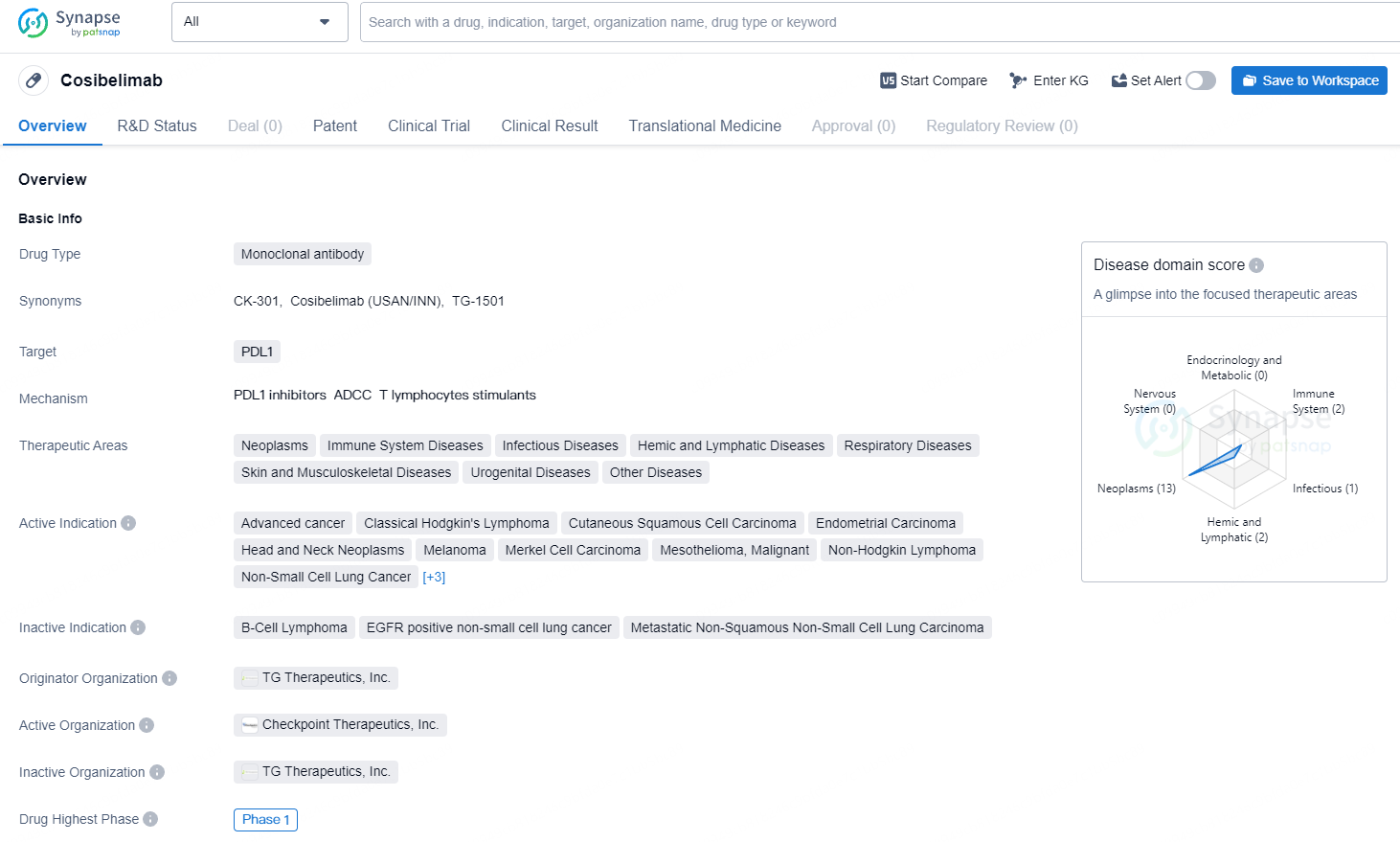
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
The resubmission of the BLA comes after Checkpoint recently reached an agreement with the FDA on its resubmission strategy, aiming to address all the issues related to approvability mentioned in the complete response letter from last December. The FDA's letter highlighted only the deficiencies found during a multi-sponsor inspection of the third-party contract manufacturing organization used by Checkpoint as approvability concerns. No issues were raised regarding the clinical data, safety, or labeling for the approvability of cosibelimab.
The BLA resubmission is underpinned by Checkpoint’s studies in selected recurrent or metastatic cancers, particularly pivotal cohorts in metastatic and locally advanced cSCC. Results related to safety and efficacy for the metastatic cSCC cohort were published in the Journal for ImmunoTherapy of Cancer in October 2023.
Furthermore, in July 2023, Checkpoint shared updated long-term data from its pivotal studies on cosibelimab in locally advanced and metastatic cSCC, indicating a deepening of response over time and higher complete response rates than previously reported.
Cosibelimab is a potentially differentiated high-affinity, fully-human monoclonal IgG1 antibody that specifically binds to PD-L1, inhibiting the interaction between PD-L1 and PD-1, as well as B7.1 receptors. The main mechanism by which cosibelimab works involves blocking the interaction between PD-L1 and its receptors PD-1 and B7.1, lifting the suppressive effects of PD-L1 on anti-tumor CD8+ T-cells and restoring the cytotoxic T-cell response. It potentially stands out from existing PD-1 and PD-L1 antibodies by maintaining high PD-L1 tumor target occupancy, which reactivates the immune response against tumors, and by having a functional Fc domain that can induce antibody-dependent cell-mediated cytotoxicity, possibly enhancing its efficacy.
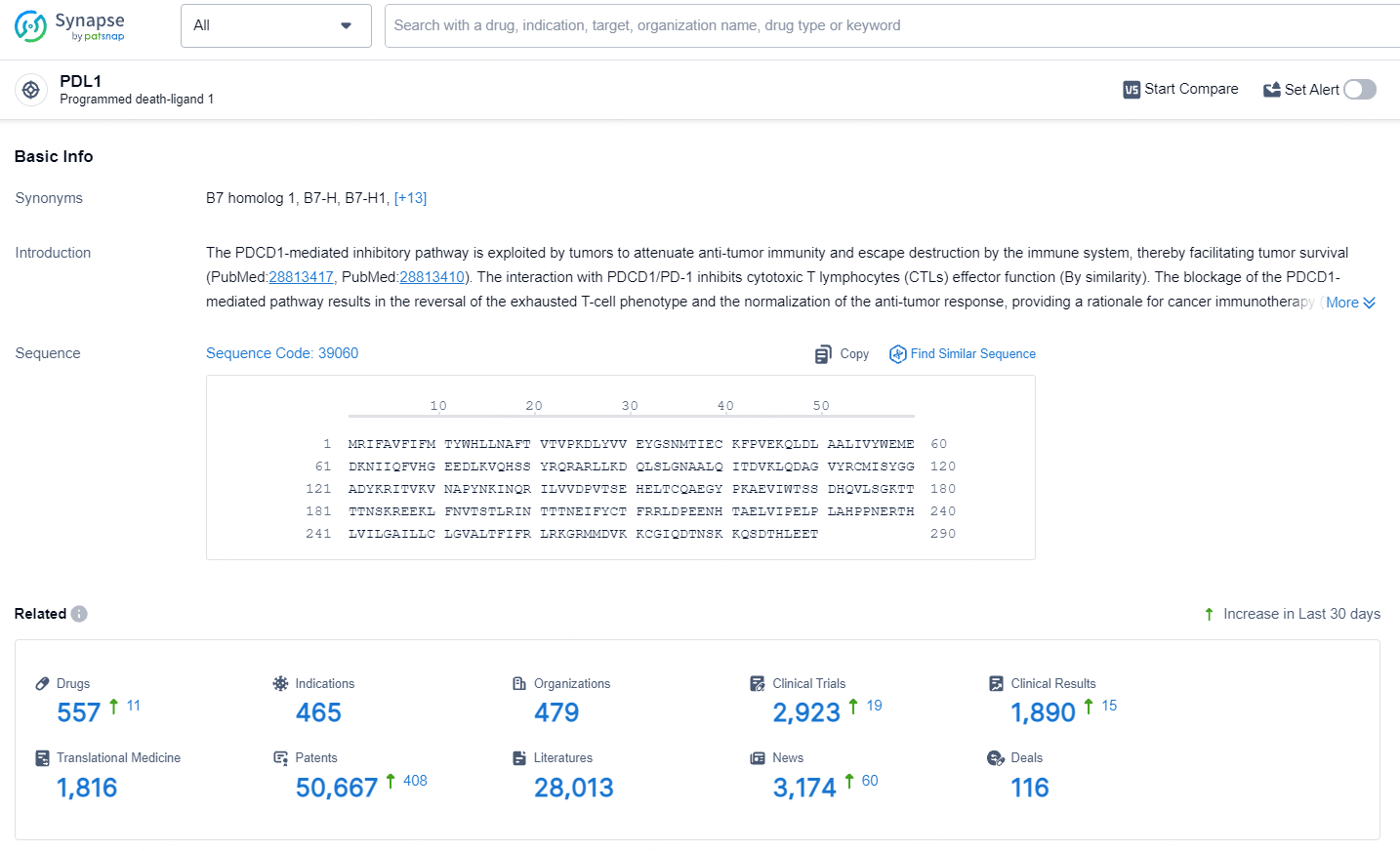
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of July 9, 2024, there are 557 investigational drugs for the PD-L1 target, including 465 indications, 479 R&D institutions involved, with related clinical trials reaching 2923, and as many as 50667 patents.
Cosibelimab has reached the highest phase of development at Phase 1 globally. This indicates that the drug is still in the early stages of clinical development and is undergoing testing to evaluate its safety, dosage, and potential effectiveness in treating the specified indications.