Epsilogen Advances Phase Ib Study of MOv18 IgE in Treatment-Resistant Ovarian Cancer with Regulatory Approval
Epsilogen, a prominent global entity in the advancement of immunoglobulin E antibodies for cancer treatment, has today revealed that the UK Medicines and Healthcare products Regulatory Agency has granted approval for the Clinical Trial Application of the Phase Ib study of MOv18 IgE.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
The Phase Ib clinical trial is scheduled to commence in late 2024 and aims to assess the effectiveness of MOv18 IgE in individuals with ovarian cancer that is resistant to platinum-based therapies.
Dr. Tim Wilson, the CEO of Epsilogen, stated, "The approval of this CTA marks a significant achievement for Epsilogen and the ongoing development of MOv18 IgE. We are eager to advance MOv18 IgE into a Phase Ib efficacy trial this year, reaffirming the potential of IgE antibodies as a novel class of cancer therapy."
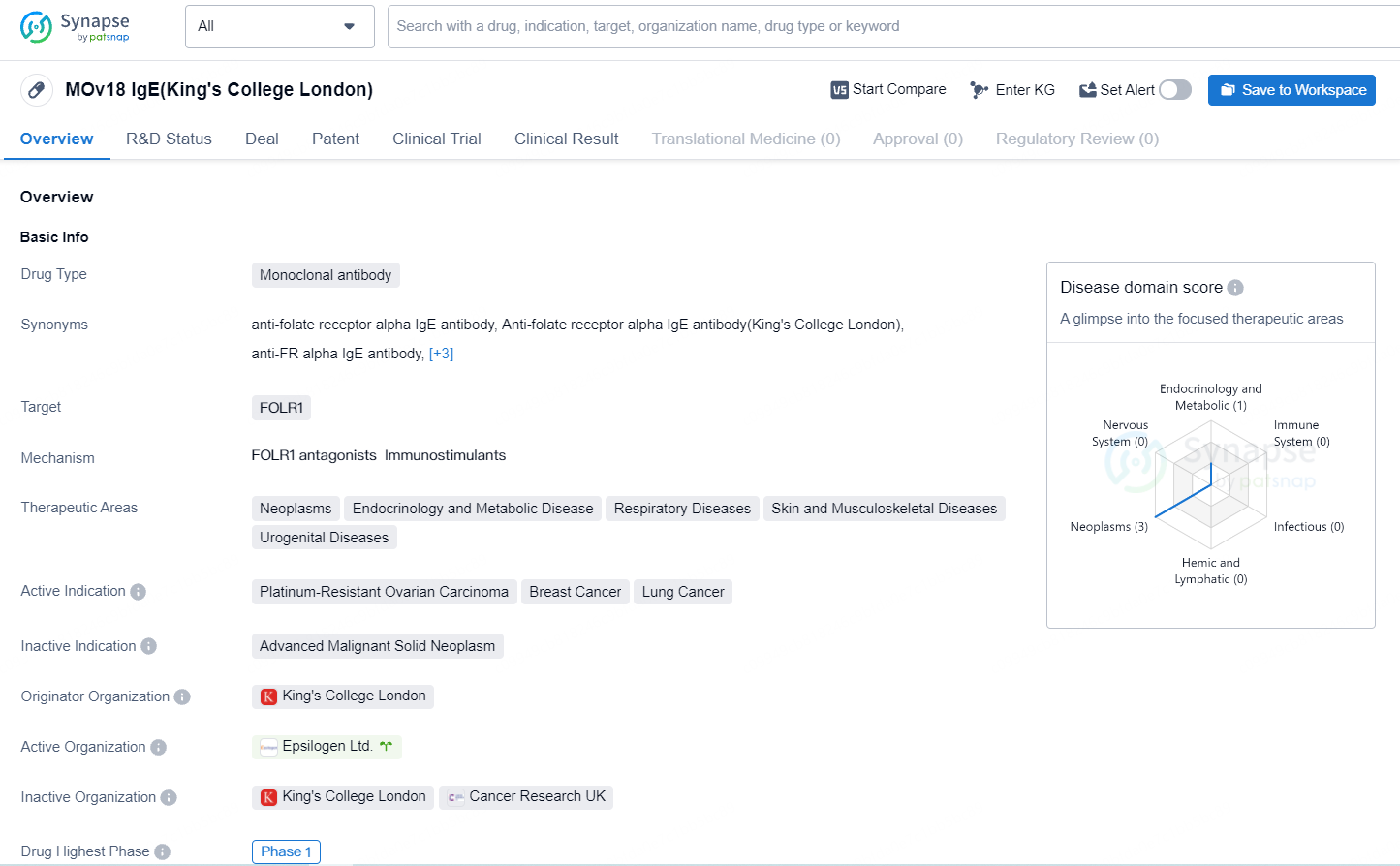
MOv18 IgE is an IgE antibody that targets the folate receptor alpha (FR alpha) antigen. FR alpha is expressed in various cancers, including ovarian, endometrial, lung, and triple-negative breast cancers. Epsilogen has already completed a Phase I study focused on the safety of MOv18 IgE in patients with platinum-resistant ovarian cancer (PROC).
The findings, published in Nature Communications, indicated that MOv18 IgE is safe and well-tolerated, with observed signs of anti-tumor activity. Earlier this year, Epsilogen and its partner Lonza announced the successful large-scale production of MOv18 IgE under Good Manufacturing Practice (GMP) conditions.
The Phase Ib study’s objective is to verify the safety and tolerability of MOv18 IgE and to demonstrate its efficacy in PROC. Post dose-escalation, an expansion cohort will be involved to make a preliminary evaluation of MOv18 IgE's anti-tumor activity at a chosen dose. Additionally, the study will assess disease progression delay and include several translational elements to further understand the role of MOv18 IgE in the patient population.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
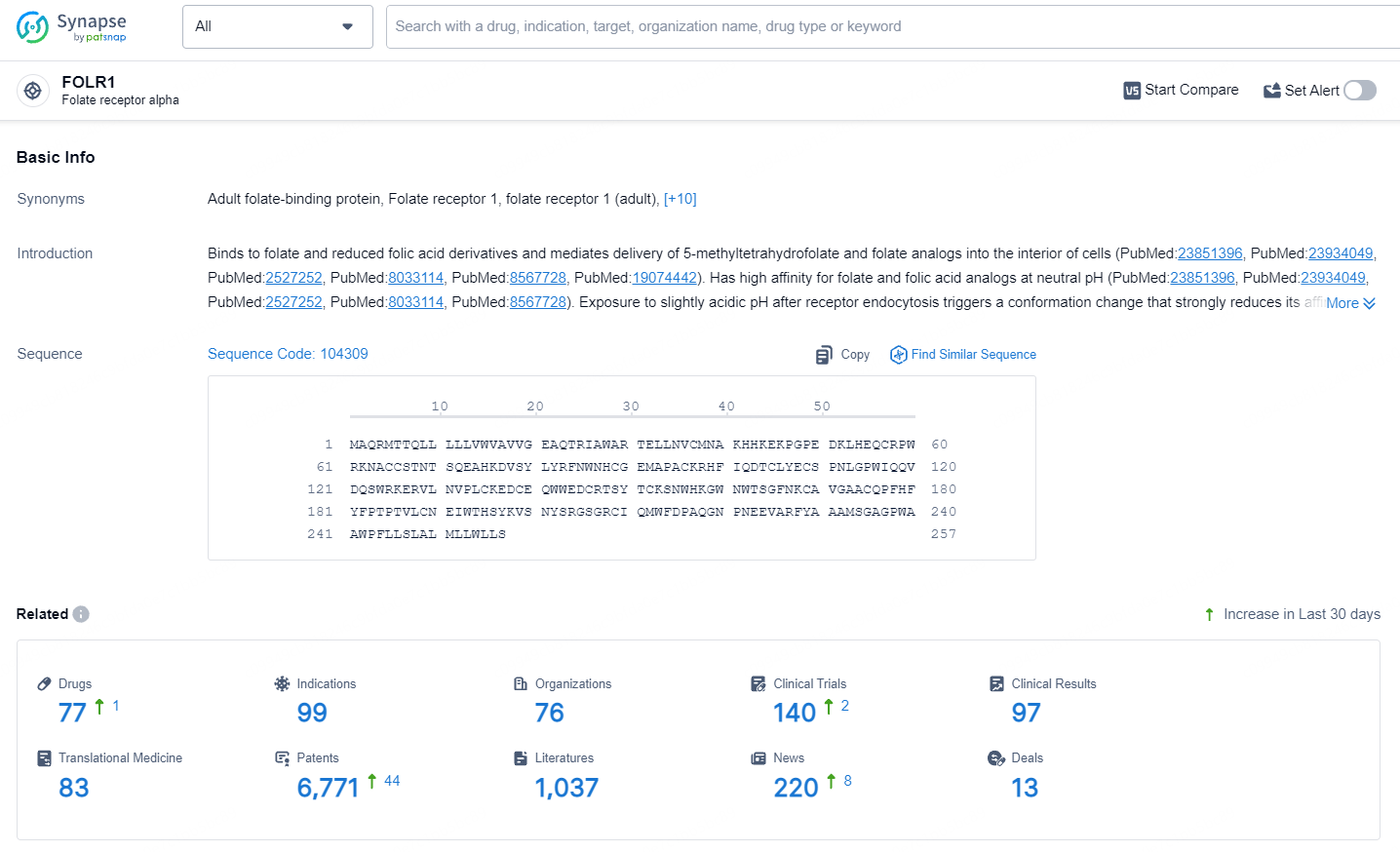
According to the data provided by the Synapse Database, As of July 9, 2024, there are 77 investigational drugs for the FOLR1 target, including 99 indications, 76 R&D institutions involved, with related clinical trials reaching 140 and as many as 6771 patents.
MOv18 IgE is a monoclonal antibody that targets FOLR1 and is being developed for the treatment of various therapeutic areas including neoplasms, endocrinology and metabolic disease, respiratory diseases, skin and musculoskeletal diseases, and urogenital diseases. MOv18 IgE represents a promising advancement in the field of biomedicine, particularly in the treatment of various types of cancer and other related therapeutic areas. As the drug progresses through clinical trials, further research and development will be necessary to determine its efficacy and safety for patients.