CinFina Pharma Presents Promising Early Findings for CIN-109 and CIN-110 at Poster Sessions
CinFina Pharma, part of the CinRx portfolio, is focused on developing effective treatment options for obesity. The company has released interim topline findings from its Phase 1 single ascending dose (SAD) trial of CIN-110, along with the final results from the multiple ascending dose (MAD) trial of CIN-109. Both studies have shown favorable safety profiles and significant reductions in body weight. CIN-110 is an innovative, long-acting PYY3-36 analog engineered to minimize gastrointestinal (GI) side effects when administered subcutaneously (SC). Meanwhile, CIN-109 is a groundbreaking, long-acting growth differentiation factor 15 (GDF-15) analog, which may help suppress appetite, sustain energy expenditure, and facilitate weight loss while protecting lean body mass.
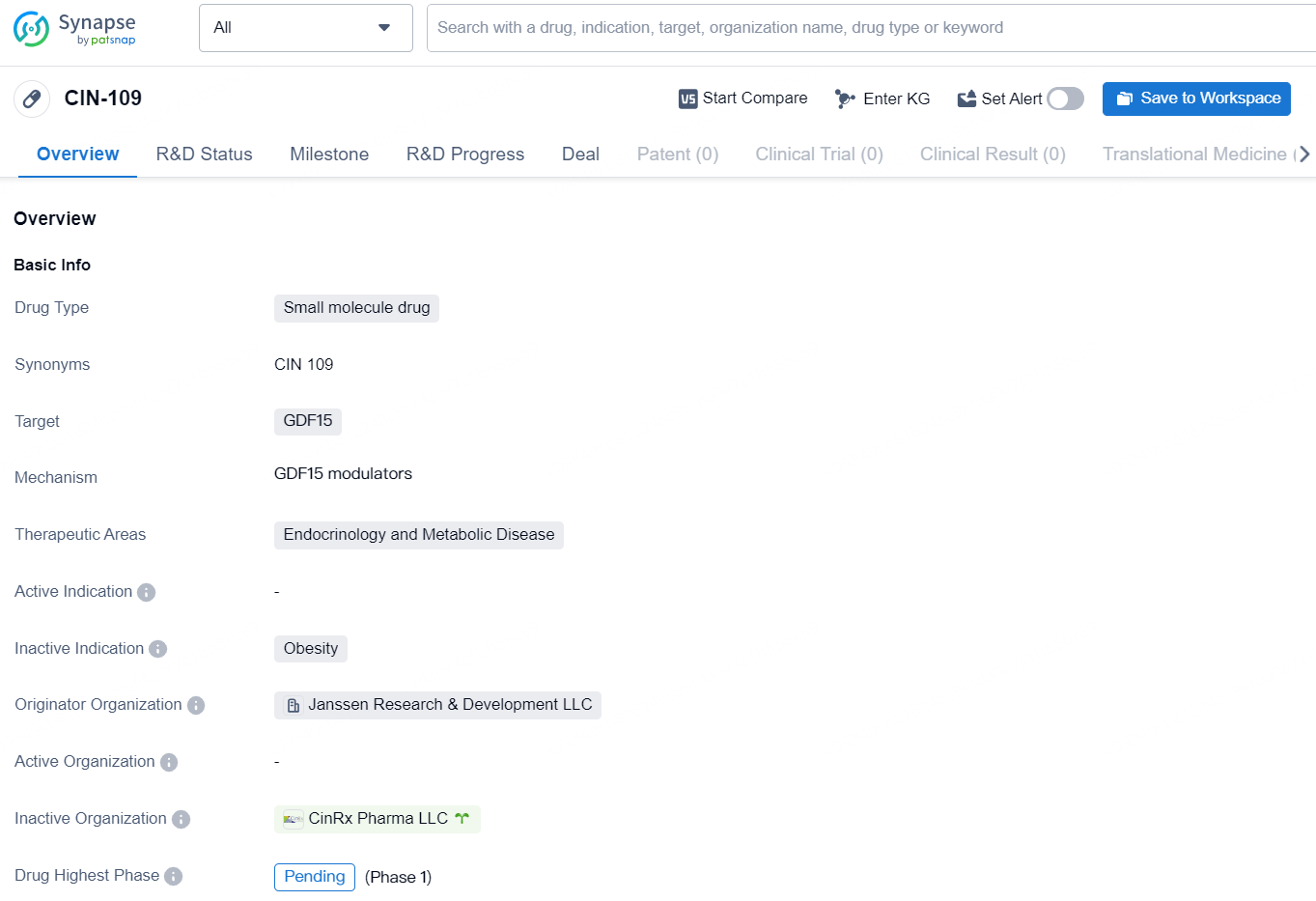
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
"Weight loss pharmaceuticals, despite their drawbacks and potential adverse effects, contribute over $6 billion in annual global revenue, with forecasts suggesting this figure could soar to $150 billion by 2030. The promising early-phase data from CIN-110 and CIN-109 lead us to believe that we can address these challenges with more advanced solutions," stated Jon Isaacsohn, M.D., Founder and CEO of CinRx. "CIN-110 stands out as a promising candidate due to its capacity to effectively and safely reduce caloric consumption while rapidly facilitating body weight loss following just one administration. Likewise, CIN-109's notable efficacy in reducing fat mass and preserving lean tissue, particularly at higher dosages, positions it as a robust long-term option."
The interim findings from the Phase 1 SAD double-blind study of CIN-110 indicated it was well tolerated and showed positive reductions in caloric intake and associated weight loss when compared to a placebo after a single subcutaneous injection. Within the first week post-dosing, participants saw food intake decrease by as much as 28%, with body weight declining by up to 1.8%. This investigation involved 24 healthy obese individuals with an average starting weight and BMI of approximately 102 kg and 34 kg/m², respectively.
CIN-110 has been specifically engineered to gradually enhance and maintain drug concentration, thereby reducing the gastrointestinal problems often linked to swift increases in PYY levels following subcutaneous administration. In total, three mild nausea cases were recorded, typically occurring within 12-48 hours post-dosing and resolving within a day, despite CIN-110 levels remaining near their peak. Pharmacokinetic evaluations indicated a gradual rise in CIN-110 levels, attaining peak concentrations usually within two to three days post-administering, with sustained levels reflecting an estimated 14-day half-life. No subjects withdrew from the CIN-110 trial due to adverse reactions, and all reported side effects were mild or moderate, with the latter being unrelated to gastrointestinal issues.
Data from the Phase 1 MAD study of CIN-109 confirmed that it was well tolerated and achieved significant weight loss in obese but otherwise healthy participants who completed the study. Conducted as a randomized, double-blind, placebo-controlled trial, participants were administered CIN-109 weekly at doses of 5 mg, 10 mg, 15 mg, 20 mg, or 40 mg, or biweekly at doses of 20 mg, 40 mg, or 60 mg. The weekly dosing group underwent treatment for four weeks, while the biweekly group was treated for eight weeks. The average weight and BMI of participants were roughly 100 kg and 35 kg/m², respectively.
Participants reported reduced caloric intake with dose-dependent decreases of up to 50%, with corresponding weight loss of up to 3.7% occurring within one to two months. The biweekly dosing regimen was better tolerated, and the majority of weight lost consisted of fat mass. No severe adverse effects related to the treatment were recorded.
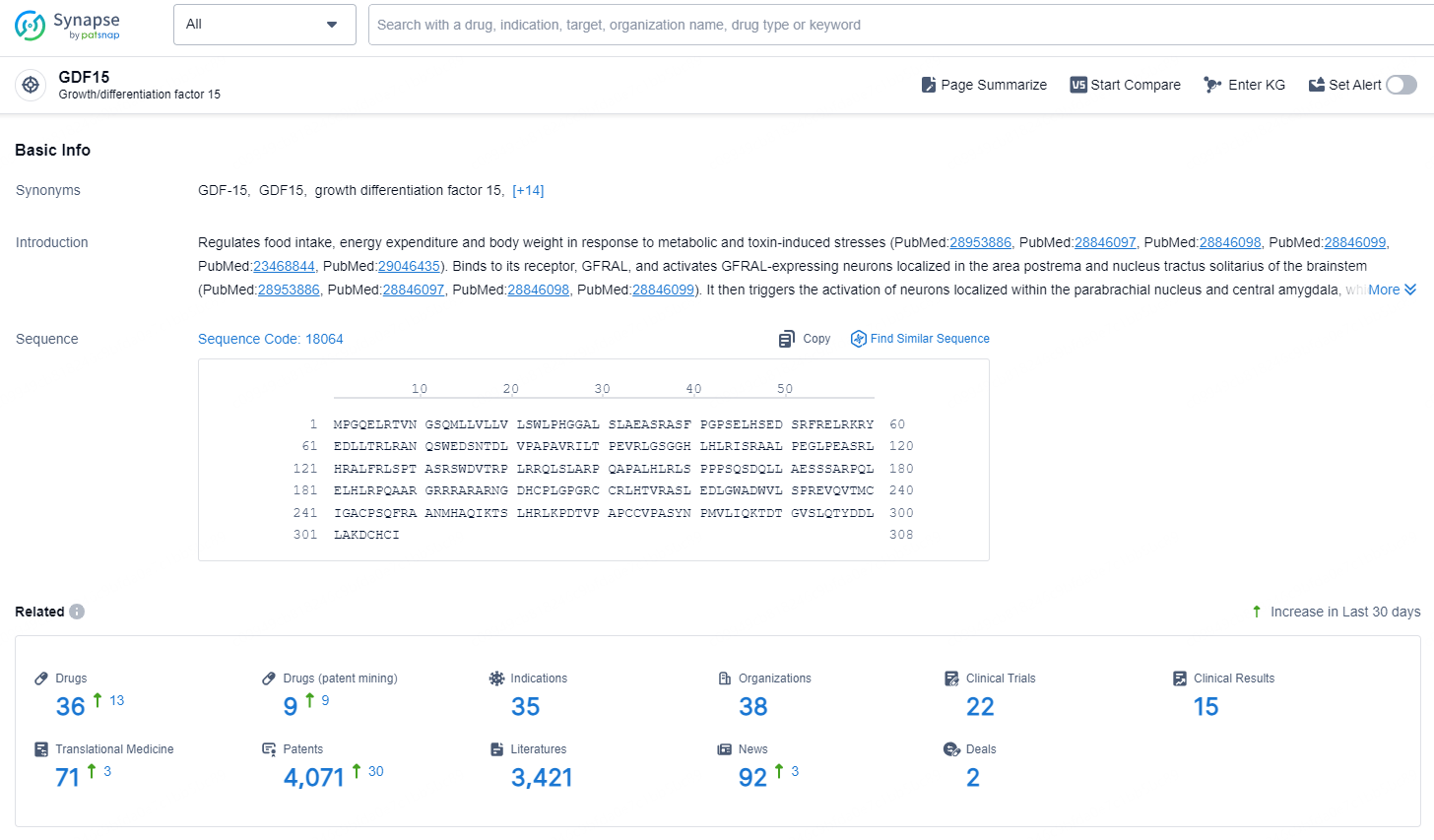
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Chemical, As of November 5, 2024, there are 36 investigational drugs for the GDF15 target, including 35 indications, 38 R&D institutions involved, with related clinical trials reaching 22, and as many as 4071 patents.
CIN-109 is a small molecule drug developed by Janssen Research & Development LLC, with a pending highest phase in the field of biomedicine. The drug targets GDF15 and is intended for therapeutic areas of Endocrinology and Metabolic Disease. As a small molecule drug, CIN-109 is characterized by its relatively low molecular weight and is designed to interact with specific target proteins within the body. In this case, the drug is tailored to target GDF15, a protein associated with various biological processes, including metabolism and energy regulation.