Clover Commences Early-Stage Human Testing for Respiratory Syncytial Virus Immunization Prospect
Clover Biopharmaceuticals, Ltd., an international biotech enterprise engaged in the commercial-scale production and advancement of groundbreaking vaccine solutions aimed at enhancing health outcomes and providing life-saving interventions globally, has disclosed the successful registration of the initial cohort of volunteers in a primary Phase Ⅰ human clinical trial. This trial is designed to assess the efficacy of its investigational vaccine, SCB-1019, directed against Respiratory Syncytial Virus (RSV). SCB-1019 is developed using the proprietary Trimer-Tag vaccine technology that forms the cornerstone of Clover's research and development efforts.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
"It brings us great satisfaction to announce that Clover has emerged as the foremost firm in China to progress an RSV prefusion-stabilized F vaccine into human trials, a testament to both our cutting-edge Trimer-Tag technology and the competency of our research team," articulated Chief Executive Officer and member of the Clover Board, Joshua Liang.
Expanding on this statement, Liang remarked, "The underserved area of RSV vaccinations, particularly for a home-grown RSV PreF vaccine yet to reach clinical trials in China, remains of critical concern. Additionally, internationally, this presents a chance for our vaccine to stand out."
SCB-1019 is a promising vaccine that targets both RSV-A and RSV-B. It is designed with a focus on the prefusion-stabilized F protein, utilizing the proven Trimer-Tag technology, complemented with unique PreF mutations for stability. Currently undergoing Phase I trials in Australia, this study is double-blind and involves a placebo control. The trial's goal is to evaluate the safety profile, tolerability, and immune response elicited by SCB-1019 when administered in various dosage strengths and formulations to younger and older adults. We anticipate initial data on safety and immune response by the latter part of 2024.
RSV is notorious for causing critical respiratory complications such as bronchiolitis and pneumonia, particularly alarming in infants and young children. By focusing on the RSV F protein for vaccine development, we aim to offer a preventive strategy against these detrimental infections, ultimately aiming to diminish the rates of sickness and death they cause.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
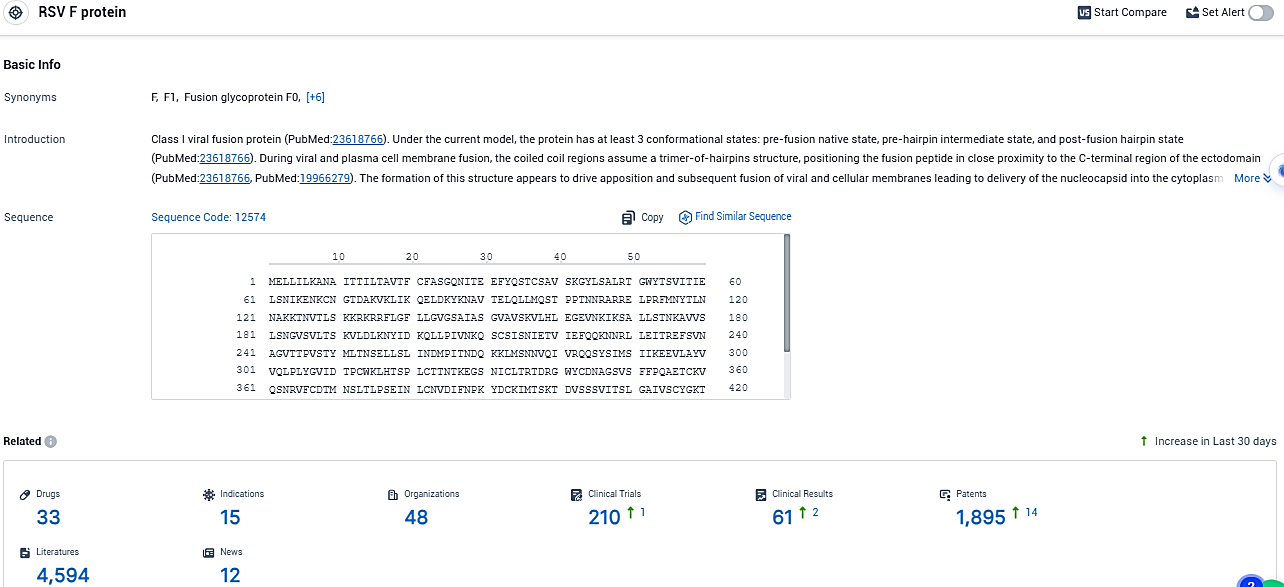
According to the data provided by the Synapse Database, As of December 19, 2023, there are 33 investigational drugs for the RSV F protein target, including 15 indications, 48 R&D institutions involved, with related clinical trials reaching 210, and as many as 1895 patents.
The RSV F antigen vaccine developed by Clover Biopharmaceuticals is a vaccine targeting the RSV F protein, with a focus on combating respiratory syncytial virus infections. With its current highest phase being Phase 1 globally and preclinical in China, the vaccine shows potential in addressing the significant health burden caused by RSV infections.