Vertex Reports Encouraging Outcomes in Early Trial of New Drug VX-548 for Alleviating Diabetes-Related Nerve Pain
Vertex Pharmaceuticals Incorporated declared favorable outcomes from its Phase 2 trial assessing various dosages of the targeted NaV1.8 inhibitor, VX-548, in individuals suffering from painful diabetic peripheral neuropathy.
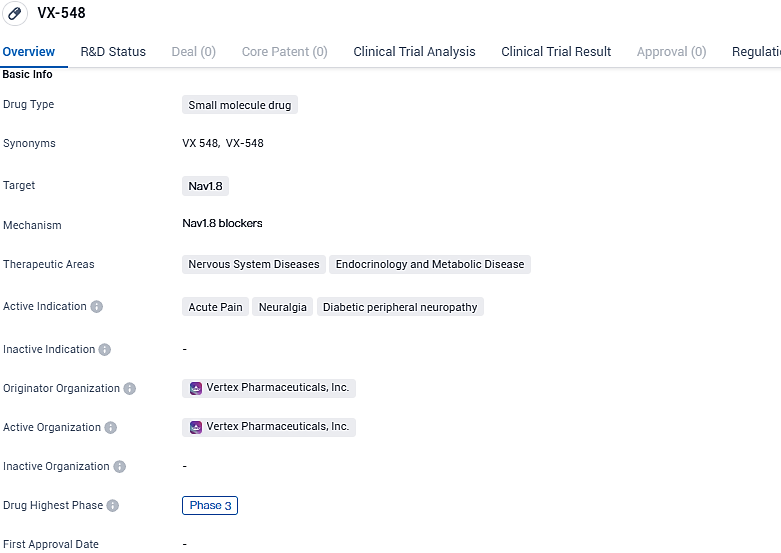
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Administering various dosages of VX-548 led to a significant and clinically relevant decrease in the key measure, which is the variation from the starting point in the weekly mean of daily pain severity, as measured on the Numeric Pain Rating Scale by the 12th week. To contextualize the efficacy of VX-548, the study included a comparator group receiving pregabalin.
Tolerance for VX-548 was observed to be favorable across the different dosage groups within the experiment. The majority of side effects were of a mild or moderate nature, and no severe side effects were attributed to VX-548.
"These outcomes are highly encouraging and contribute to the growing evidence supporting the safety and effectiveness of VX-548. They further confirm the analgesic properties of NaV1.8 inhibitors," expressed Carmen Bozic, M.D., Vertex's Executive Vice President of Global Medicines Development and Medical Affairs, and Chief Medical Officer.
"With the positive balance between benefits and risks observed for VX-548 in this trial, we are proactively progressing this non-opioid investigational therapy through to the next phase of clinical trials for painful diabetic neuropathy, aiming to revolutionize current treatment standards for neuropathic pain, which are currently inadequate. We are also on schedule to obtain Phase 3 clinical trial results for VX-548 in acute pain by early 2024," further stated Carmen Bozic.
"The VX-548 Phase 2 DPN study's findings are exceptional and suggest a favorable safety and efficacy profile," remarked Roy Freeman, M.D., Professor of Neurology and Director of the Center for Autonomic and Peripheral Nerve Disorders at Beth Israel Deaconess Medical Center. "This represents an important development in the treatment of pain."
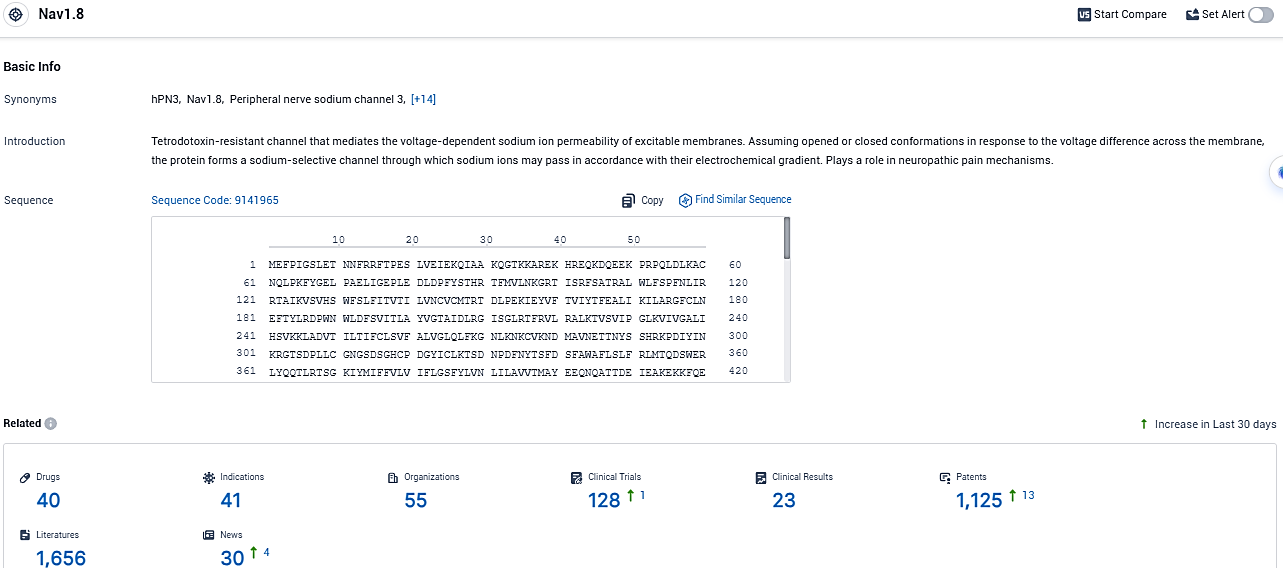
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of December 19, 2023, there are 40 investigational drugs for the NaV1.8 target, including 41 indications, 55 R&D institutions involved, with related clinical trials reaching 128, and as many as 1125 patents.
VX-548 is an investigational oral, selective NaV1.8 inhibitor that is highly selective for NaV1.8 relative to other NaV channels. VX-548 is one of the most recent molecules to enter clinical development from Vertex’s portfolio of NaV1.8 inhibitors.