Clover Reveals Encouraging Initial Study Outcomes of SCB-219M in Managing Low Platelet Counts Caused by Cancer Drugs
Clover Biopharmaceuticals, Ltd., has disclosed encouraging initial results for safety, effectiveness, and drug metabolism and distribution assessment in an early-stage clinical study. The trial investigates SCB-219M, a cutting-edge bispecific Fc-fusion protein that acts as a thrombopoietin receptor agonist (TPO-RA) designed using Chinese hamster ovary (CHO) cell expression systems. This novel protein is aimed at alleviating thrombocytopenia caused by chemotherapy in oncology patients.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
To date, all cancer patients included in our study, who were treated with their standard chemotherapy plus a one-time injection of SCB-219M under the skin, have consistently shown either stable or increased levels of platelets exceeding 75 x 10^9/L after the first week. This beneficial effect persisted for a minimum duration of three weeks. Historically, when these same participants underwent their customary chemotherapy without enrolling in our study, their platelet counts uniformly fell below 75 x 10^9/L within a period ranging from one to three weeks.
The promising initial outcomes in terms of both efficacy and the way SCB-219M is broken down and distributed in the body suggest that doses every two weeks or more may be feasible. With additional confirmation, this dosing frequency could be conveniently aligned with the typical 2-3 week cycles of a patient's chemotherapy treatment. To this point, the safety profile of SCB-219M has been satisfactory, without detection of any severe negative events or toxicity that would limit dosing levels.
Commenting on the SCB-219M project, Dr. Yongsheng Wang, the Associate Director of the Cancer Center at West China Hospital, Sichuan University, and the lead researcher on this phase of the trial said, "The initial findings from Phase Ⅰ of the SCB-219M trial are promising, and we are optimistic about the continuing analysis of SCB-219M, as it might address the substantial need for better management of chemotherapy-induced thrombocytopenia (CIT) for those undergoing cancer treatment."
Dr. Peng Liang, Chairman and Chief Scientific Officer of Clover and the creator of SCB-219M, stated with enthusiasm, "We are excited to release these initial findings from Phase Ⅰ for SCB-219M, which reveal swift and sustained results with regards to efficacy and a satisfactory safety record. When compared with the current biologic treatments available for CIT in China, the observed long-term effects and pharmacokinetic data gathered thus far on SCB-219M may provide a more practical dosing schedule that can be tailored to fit the chemotherapy cycles of individual patients."
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
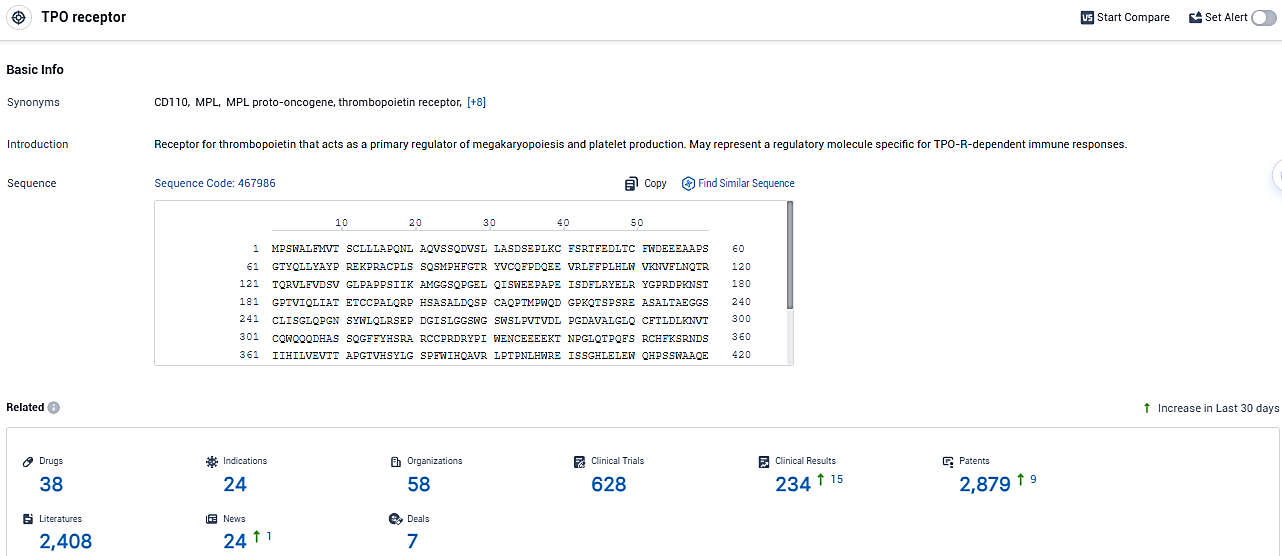
According to the data provided by the Synapse Database, As of January 4, 2024, there are 38 investigational drugs for the TPO receptor, including 24 indications, 58 R&D institutions involved, with related clinical trials reaching 628, and as many as 2879 patents.
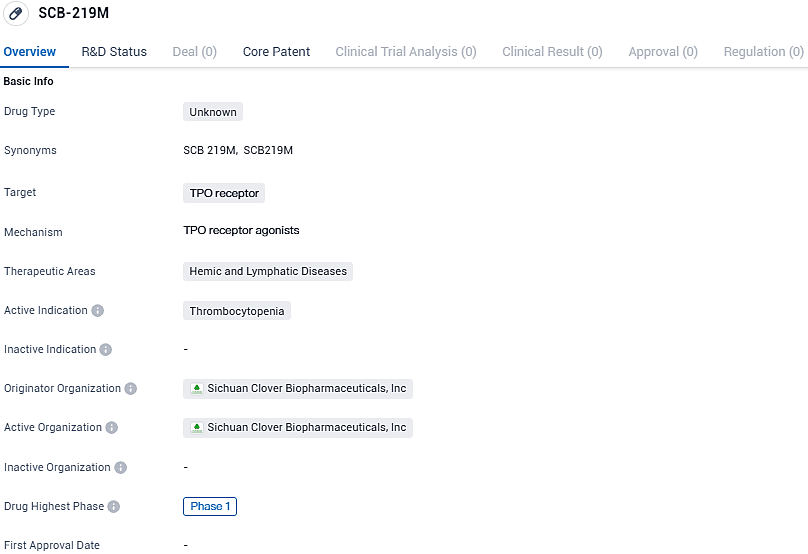
SCB-219M targets the TPO receptor and is intended for the treatment of thrombocytopenia, a condition characterized by low platelet count. Currently, SCB-219M is in Phase 1 of clinical trials both globally and in China. Further research and development will be necessary to determine the drug's efficacy and potential market impact.