Context Therapeutics Acquires Phase 1-ready T Cell Engager CT-95
Context Therapeutics Inc., a biopharmaceutical firm focused on developing treatments for solid tumors, has publicized the acquisition of CT-95, previously the property of Link Immunotherapeutics, Inc. The CT-95 is a mesothelin x CD3 T cell engaging bispecific antibody boasting first-in-class potential, which has obtained Investigational New Drug approval from the U.S. Food and Drug Administration. CT-95's Phase 1 clinical trial is slated to begin in the first quarter of 2025.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
 Martin Lehr, CEO of Context, expressed his excitement about the inclusion of CT-95, previously recognized as LNK101, into their development portfolio. He stated, “The acquisition of this MSLN x CD3 bispecific antibody aligns perfectly with our strategy of expanding our pipeline with TCE assets aimed at treating solid tumors. Context chose MSLN as a key target because of its significant presence in underserved solid tumors such as ovarian, lung, and pancreatic cancers. Given the promising preclinical data accumulated thus far, we are confident that CT-95 could be both a pioneering and leading MSLN-targeting TCE.”
Martin Lehr, CEO of Context, expressed his excitement about the inclusion of CT-95, previously recognized as LNK101, into their development portfolio. He stated, “The acquisition of this MSLN x CD3 bispecific antibody aligns perfectly with our strategy of expanding our pipeline with TCE assets aimed at treating solid tumors. Context chose MSLN as a key target because of its significant presence in underserved solid tumors such as ovarian, lung, and pancreatic cancers. Given the promising preclinical data accumulated thus far, we are confident that CT-95 could be both a pioneering and leading MSLN-targeting TCE.”
Mr. Lehr added, “CT-95 has received IND clearance, and we are eager to swiftly advance it into clinical trials. We plan to finance both its acquisition and progression through the dose escalation phase of a Phase 1 clinical trial using Context’s current cash resources.”
MSLN is a membrane protein that is overexpressed in many cancer types while showing limited expression in normal tissues. A key challenge in the development of MSLN-targeted therapies has been the shed MSLN sink present in blood and the tumor microenvironment.
CT-95 is a fully humanized bispecific TCE utilizing an IgG-scFv architecture with an effector-silenced IgG1 backbone. It demonstrates low affinity but high avidity for membrane-bound MSLN, effectively reducing the influence of the shed sink. CT-95 is being developed as a treatment for advanced cancers associated with MSLN expression.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
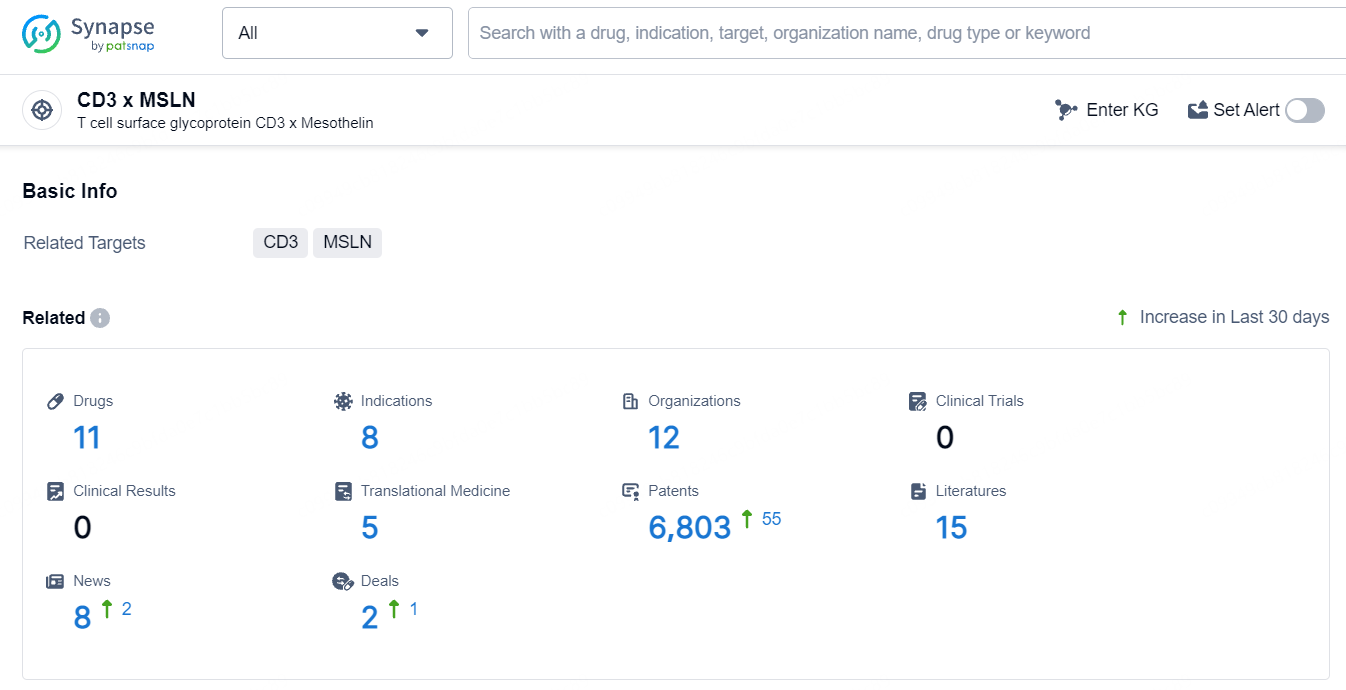
According to the data provided by the Synapse Database, As of July 16, 2024, there are 11 investigational drugs for the CD3 and MSLN target, including 8 indications, 12 R&D institutions involved, and as many as 6803 patents.
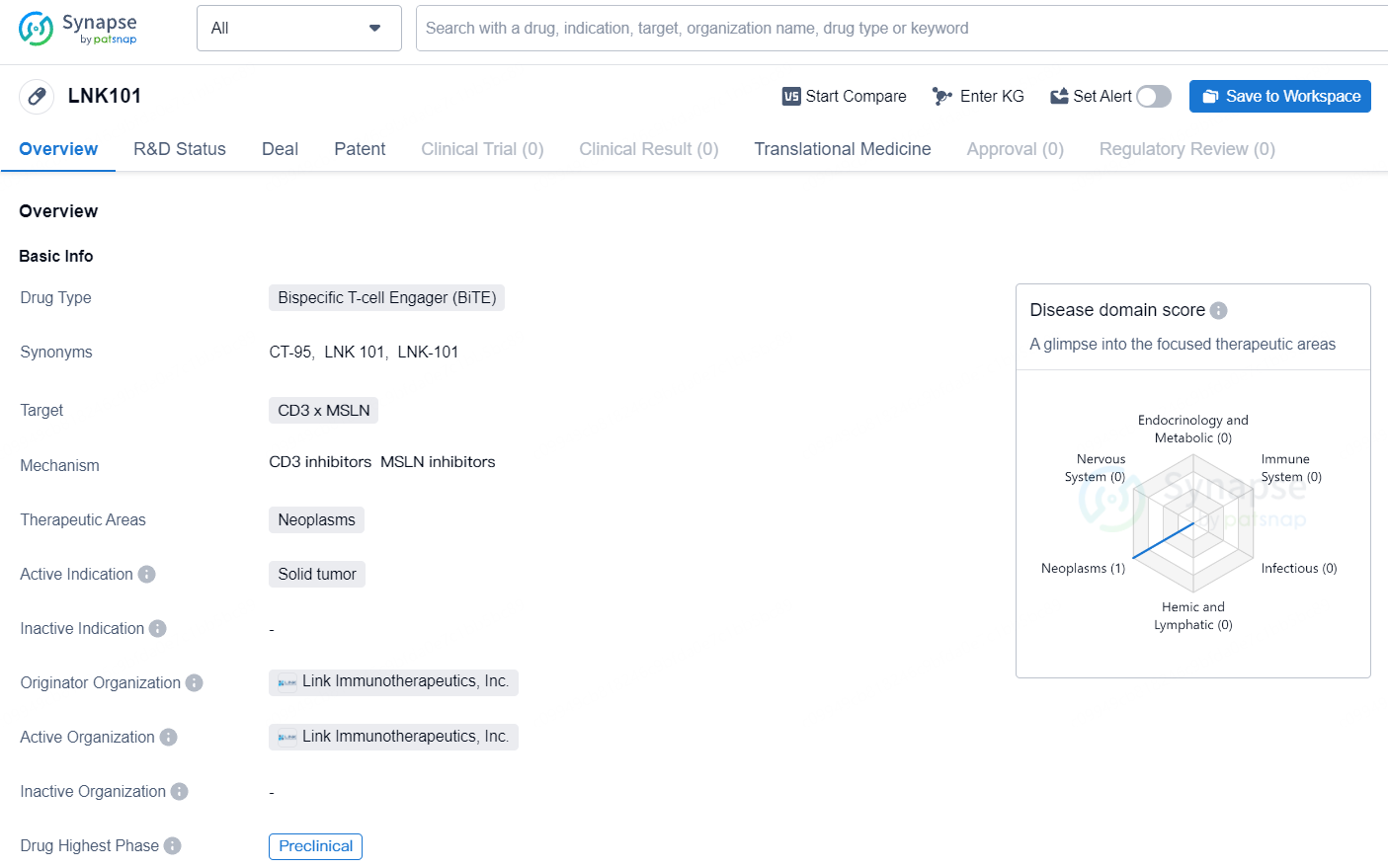
LNK101 shows promise as a potential therapeutic option for the treatment of solid tumors. However, further research and clinical trials will be necessary to evaluate its safety and efficacy in human patients. As a preclinical stage drug, LNK101 is still in the early phases of development, and its progress towards clinical trials and approval will be important milestones to watch in the future.