Context Therapeutics Initiates Phase 1 Trial for CTIM-76 Following FDA IND Approval
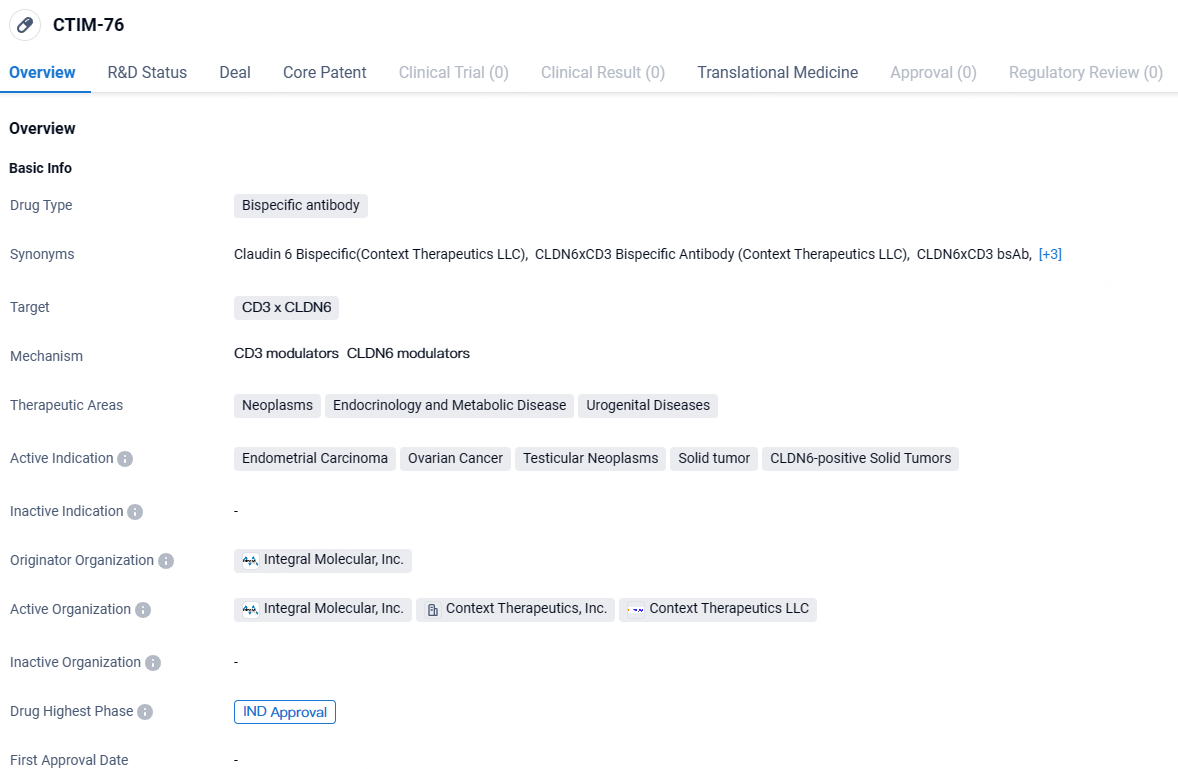
Context Therapeutics Inc., engaged in developing treatments for solid tumors, disclosed that its application for the Investigational New Drug, CTIM-76, has been approved by the U.S. Food and Drug Administration. CTIM-76 is a bispecific antibody targeting Claudin 6 ("CLDN6") and CD3 for T cell engagement.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The IND has greenlit a Phase 1 clinical study for CTIM-76, focusing on dose escalation and expansion in patients with CLDN6-positive gynecologic and testicular cancers. The company expects to begin enrolling patients in the initial dose escalation segment of this trial by mid-2024.
Context's CEO, Martin Lehr, commented, "The FDA's approval of our IND is a significant milestone for our company, enabling us to launch the Phase 1 trial for our promising CLDN6-targeted therapy. We are optimistic about administering CTIM-76 to our first patient in the near future and are confident in the company's capability to reach critical milestones in this program."
The upcoming Phase 1 trial for CTIM-76 is designed as an open-label study that will assess both dose escalation and expansion to evaluate the therapeutic's safety and efficacy in treating advanced or metastatic ovarian, endometrial, and testicular cancers positive for CLDN6. This trial aims to engage up to 70 participants and will assess outcomes including safety, tolerability, pharmacokinetics, overall response rate, response duration, and disease control rate.
CTIM-76, a CLDN6 x CD3 T cell engaging bispecific antibody, targets diverse solid tumors including certain ovarian, endometrial, lung, gastric, and testicular cancers. Ongoing preclinical studies highlight CTIM-76's potential advantages such as feasible dosing schedules, low risk of immunogenicity, and scalable production, making it a viable option for a substantial patient population eligible for this therapy.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
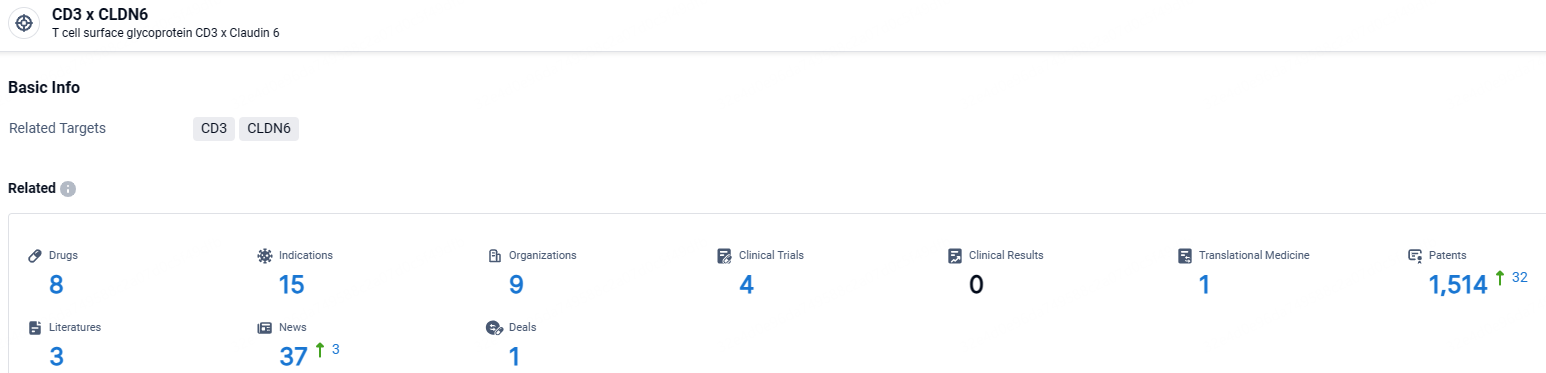
According to the data provided by the Synapse Database, As of May 9, 2024, there are 8 investigational drugs for the CD3 and CLDN6 targets, including 15 indications, 9 R&D institutions involved, with related clinical trials reaching 4, and as many as 1514 patents.
CTIM-76 targets the CD3 x CLDN6 receptors and is indicated for the treatment of endometrial carcinoma, ovarian cancer, testicular neoplasms, solid tumors, and CLDN6-positive solid tumors. The drug has reached the highest phase of development, which is IND approval, indicating its readiness for clinical trials.