What ADC-related progress will Alphamab Biopharmaceuticals reveal at the ASCO conference?
Alphamab Biopharmaceuticals is set to debut clinical research data from China at the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting, relating to the treatment of HER2-expressing solid tumors using their novel bispecific HER2-targeting antibody-drug conjugate (ADC), JSKN003.

JSKN003 is a novel ADC targeting two distinct epitopes of HER2, independently developed by Alphamab Biopharmaceuticals utilizing its proprietary glycan-based site-specific conjugation platform. It acts by binding to the HER2 on the surface of tumor cells and releasing a topoisomerase I inhibitor via HER2-mediated endocytosis, thereby exerting anti-tumor effects. Preclinical studies have shown that JSKN003 offers superior serum stability, enhanced bystander killing effect, and comparable tumor-killing activity to similar drugs, effectively broadening the therapeutic window. Presently, multiple clinical trials of JSKN003 are progressing smoothly in Australia and China, including key clinical studies in China focusing on the treatment of advanced, low HER2-expressing breast cancer.
JSKN003 boasts advantages such as targeting two different epitopes of HER2, glycan-based site-specific conjugation, improved serum stability, and a broader therapeutic window. Data from the Phase I clinical trial in Australia, unveiled at the American Association for Cancer Research (AACR) Annual Meeting in April, demonstrated promising tolerability, safety, and preliminary anti-tumor activity. Multiple clinical trials of JSKN003 are successfully underway in Australia and China, with active progression in key clinical studies targeting late-stage, low HER2-expressing breast cancer in China.
JSKN003-102 (NCT05744427) is an ongoing Phase I (dose escalation and expansion) and Phase II (cohort expansion) study conducted in China. It enrolls patients histologically confirmed to express HER2 (IHC ≥ 1+) or those with HER2-mutant tumors failing previous systemic treatments, aiming to evaluate the safety, pharmacokinetics (PK), and preliminary anti-tumor activity of JSKN003 as a monotherapy, and to determine the maximum tolerated dose (MTD) or Phase II recommended dose (RP2D). The results from the Phase I study will be disclosed at the upcoming ASCO meeting.
Founded in 2015, Alphamab Biopharmaceuticals specializes in the research, development, production, and commercialization of biologic anti-cancer drugs. The company has established proprietary platforms for protein/antibody engineering, antibody screening, and multimodal/multifunctional antibody modifications, encompassing a range of innovative anti-cancer drugs including single domain antibodies, monoclonal antibodies, multifunctional antibodies, and antibody conjugates.
How to search for and analyze the development progress of ADC pharmaceuticals?
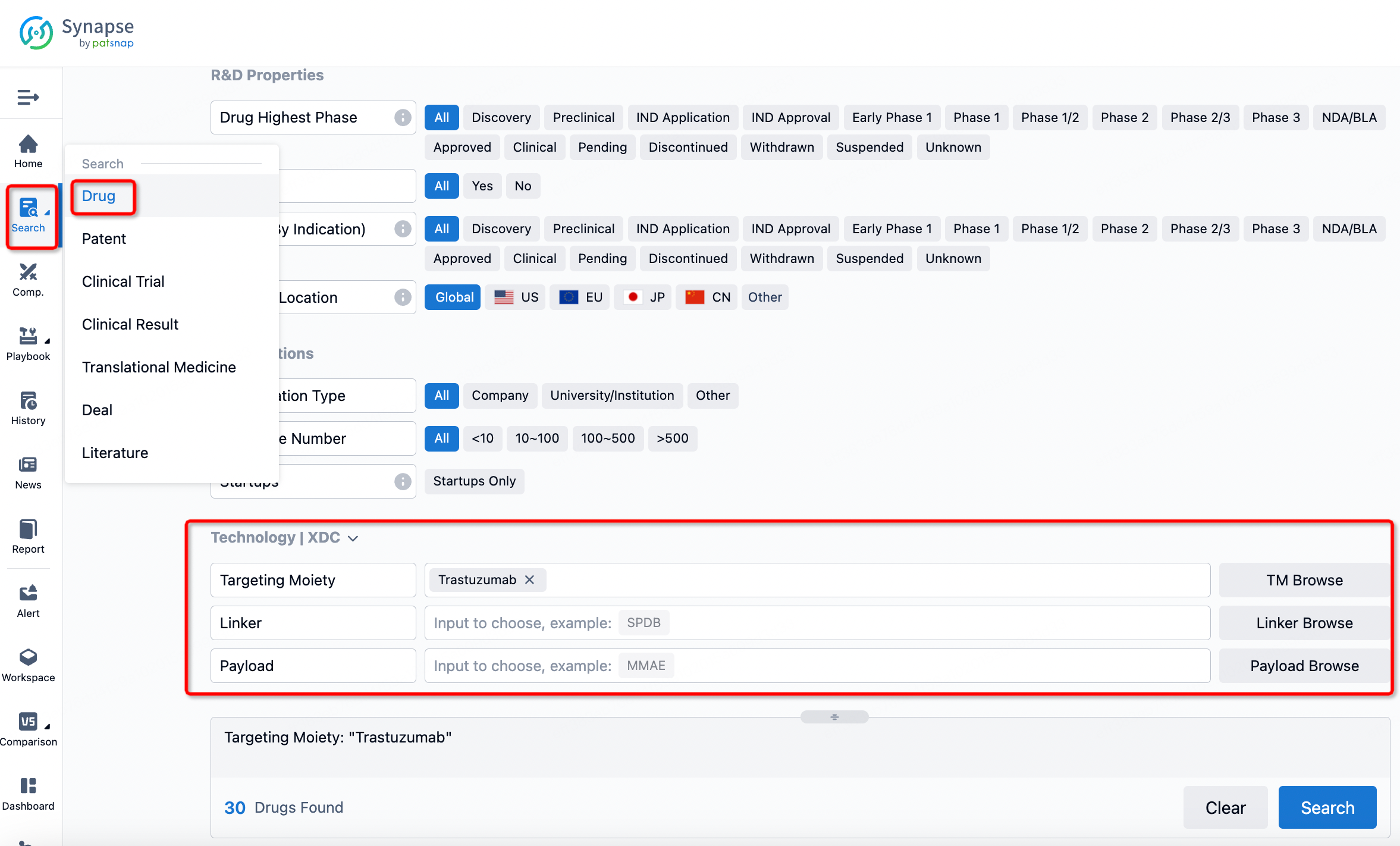
If you want to learn about the latest developments in ADC drugs, you can use the drug search module of the Synapse database. This module supports searching for ADC drugs by classification through Targeting Moiety, Linker, and Payload.
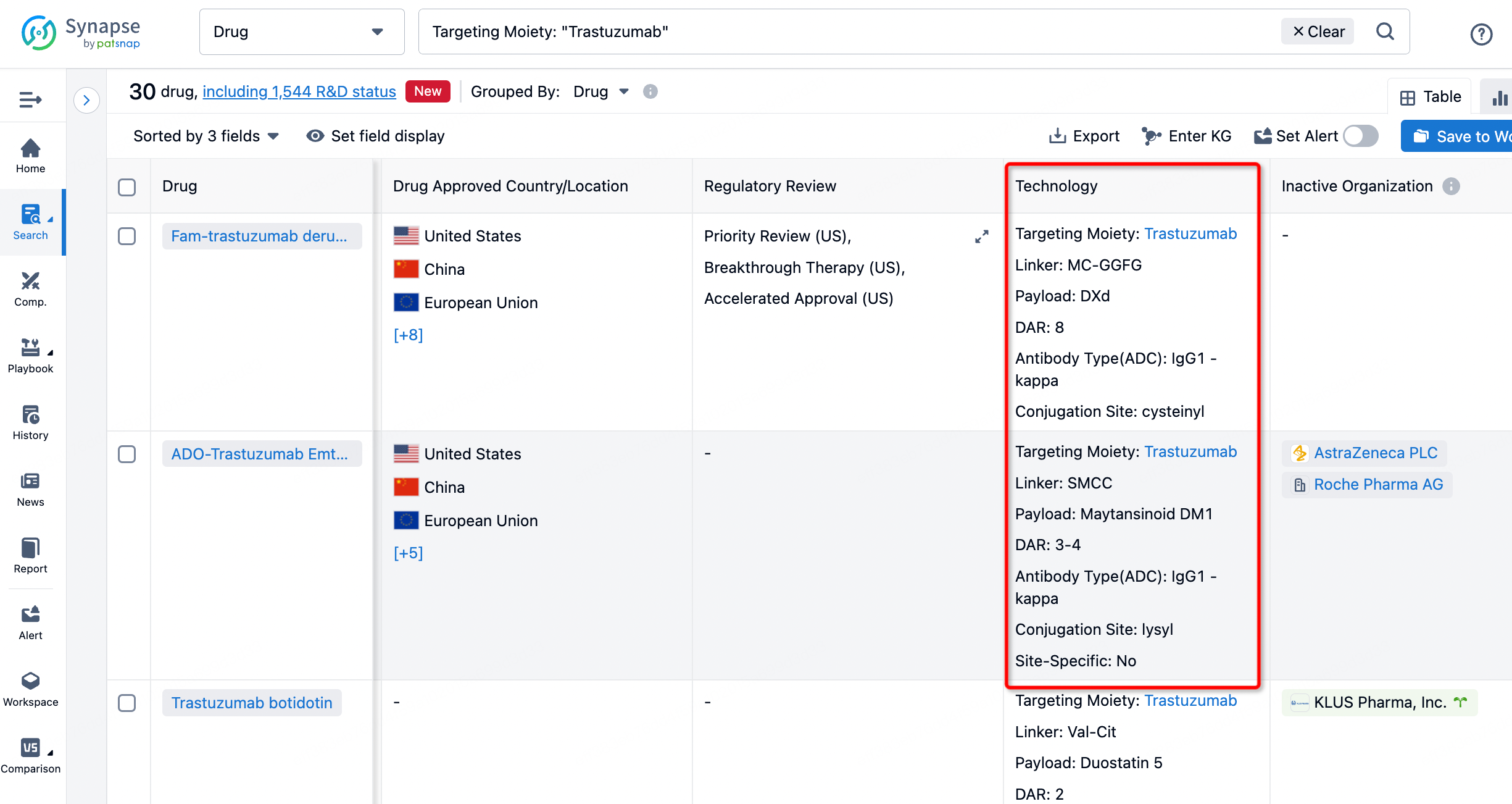
On the search results page, you can easily review information related to the ADC's technical category of the drugs.
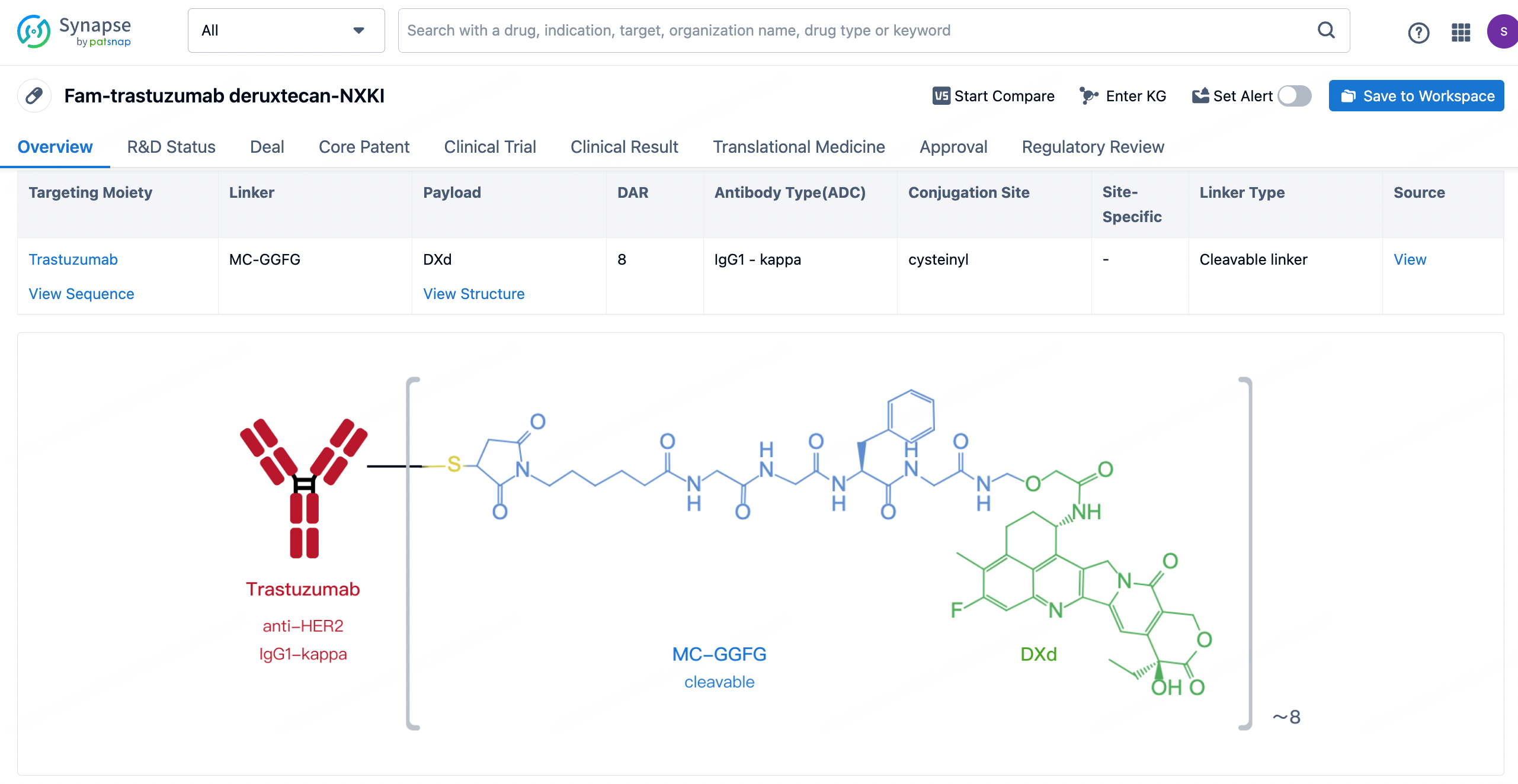
After clicking to enter the drug details page, you can also effortlessly obtain structural information about the ADC drug.
Click on the image below to embark on a brand new journey of drug discovery!