Cullinan Oncology's CLN-619: FDA Greenlights Initial Trials for Novel MICA/B Antibody in Advanced Multiple Myeloma
Cullinan Oncology, Inc., a firm specializing in biopharmaceuticals with a commitment to developing targeted cancer treatments that are independent of modality, has disclosed that the U.S. Food and Drug Administration has approved their request to commence clinical trials for CLN-619, a novel therapy for patients with recurrent or resistant multiple myeloma.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
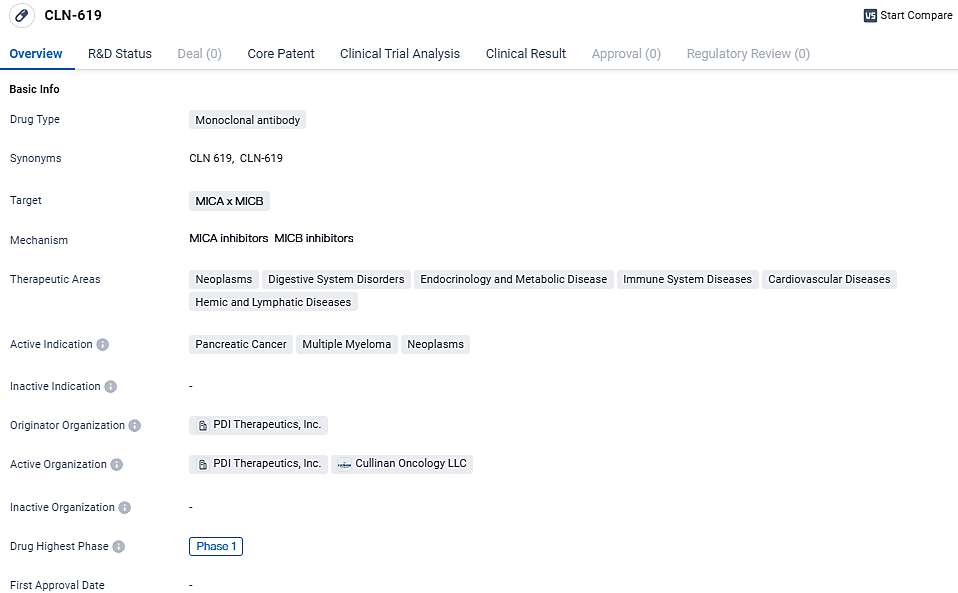
As a potentially groundbreaking humanized IgG1 monoclonal antibody, CLN-619 uniquely targets the stress-responsive molecules MICA and MICB that are prevalent across numerous solid tumors and blood cancers. The launch of a Phase 1 clinical investigation for CLN-619, entailing both the ramp-up of dosage and the broadening of the cohort under study, is on the horizon.
"Despite advances, multiple myeloma remains a disease that defies cure, with patients typically suffering from back-to-back relapses. As the disease recurs, the effectiveness of therapies tends to diminish, accentuating the demand for innovative treatment strategies. A typical scenario involves multiple myeloma cells circumventing the immune system by shedding MICA and MICB—a process that underscores the need for new approaches," stated Chief Medical Officer Jeffrey Jones, MD, MPH, MBA, from Cullinan Oncology.
Dr. Jones also noted, "This initial clinical Phase 1 evaluation will scrutinize the effects of CLN-619 on individuals battling multiple myeloma. The encouraging safety data thus far for CLN-619 bolsters our confidence in the possibility of its integration with a range of established treatments. As we extend our investigations to touch on both solid and blood-related cancers, our goal is to truly capture the comprehensive capabilities of CLN-619 in filling significant gaps in clinical care."
Currently under assessment in an active Phase 1 clinical study, CLN-619 demonstrates promise both as an independent therapy and when paired with the drug pembrolizumab for patients with solid tumors.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
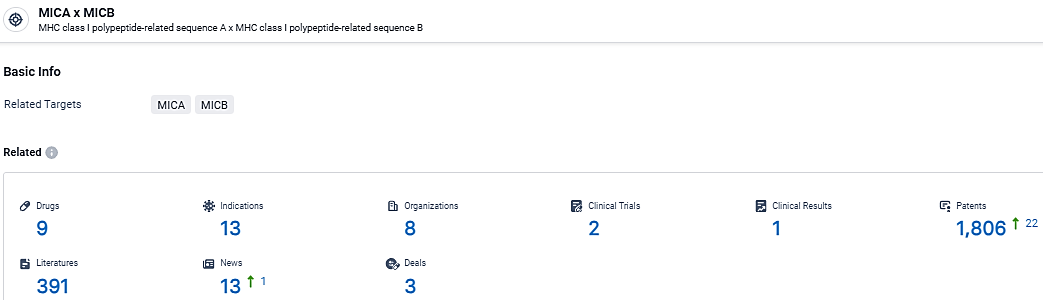
According to the data provided by the Synapse Database, As of March 5, 2024, there are 9 investigational drugs for the MICA and MICB target, including 13 indications, 8 R&D institutions involved, with related clinical trials reaching 2, and as many as 1806 patents.
CLN-619 targets MICA x MICB proteins and has the potential to be used in the treatment of various diseases, including pancreatic cancer, multiple myeloma, and neoplasms. The drug is currently in Phase 1 of clinical development, and further research is needed to determine its efficacy.