Cure Genetics Unveils Positive Safety and Efficacy of CAR-NKT Therapy CGC729 in RCC at ASGCT 2024
Cure Genetics revealed safety and efficacy outcomes for their CAR-NKT treatment, CGC729, in patients with relapsed and refractory metastatic renal cell carcinoma during a talk at the 27th Annual Meeting of the American Society for Gene & Cell Therapy held in Baltimore. This inaugural human trial employing CAR-NKT therapy for R/R mRCC showed a favorable safety profile alongside promising anti-tumor effects.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
This Phase I dose-escalation clinical trial was implemented at Fudan University Shanghai Cancer Center, employing a single-arm, 3+3 design to assess the safety and efficacy of CGC729 in treating R/R mRCC patients who had undergone a minimum of two prior therapy lines. By April 2024, five subjects were enrolled and received a single dose of CGC729. Prior to infusion, all patients experienced lymphodepletion.
Safety Assessment: As of April 11, 2024, all five patients exhibited no dose-limiting toxicities. The prevalent adverse effects were lymphodepletion-induced neutropenia, thrombocytopenia, and leukopenia. One patient at dose level 1 (DL1) experienced grade 2 cytokine release syndrome, which subsided within 24 hours; this patient also encountered grade 2 immune effector cell-associated neurotoxicity syndrome, which improved swiftly following symptomatic treatment.
Efficacy Assessment: Among four evaluable patients, the overall response rate was recorded at 50%. For CD70 positive patients, the ORR was 66.7%, with two patients achieving a partial response. CGC729 demonstrated promising efficacy with significant anti-tumor effects. The longest duration of response extends to nine months after treatment. Furthermore, pharmacokinetic data indicated substantial expansion of CGC729 in all patients, regardless of their tumor's CD70 expression level, with persistence in the blood for up to 20 weeks.
Conclusion: The interim analysis of this first-in-human study indicates that the anti-CD70 CAR-NKT (CGC729) has a favorable safety profile and strong anti-tumor activity. It shows promise as a future therapeutic option for R/R mRCC.
CGC729 is an autologous anti-CD70 CAR-NKT with a unique mechanism distinct from T cells and NK cells. Firstly, CGC729 integrates adaptive and innate immunity, utilizing multiple tumor-killing mechanisms targeting CD70, CD1d, and frequently found stress ligands in RCC. Additionally, CGC729 can modify the TME by promoting macrophage differentiation and countering TGF-β inhibition.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
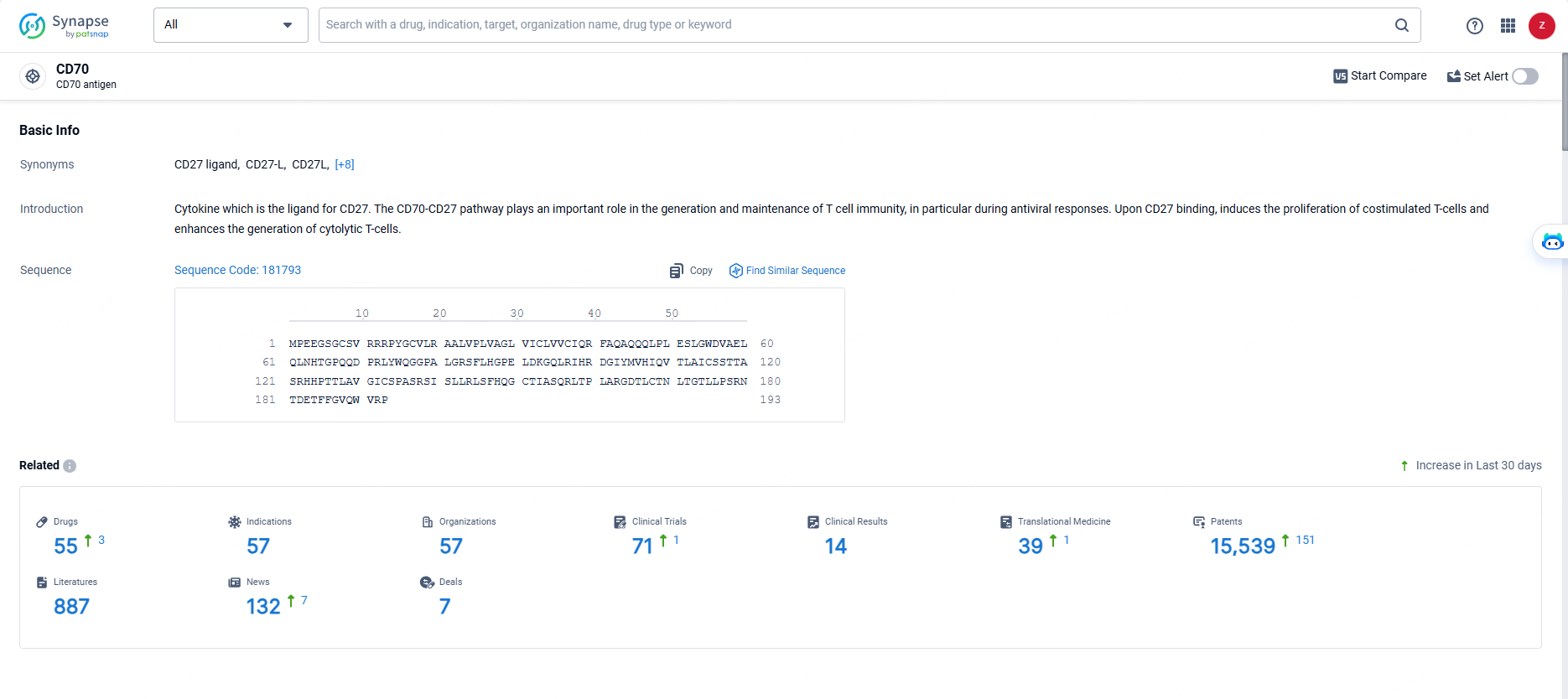
According to the data provided by the Synapse Database, As of May 29, 2024, there are 55 investigational drugs for the CD70 targets, including 57 indications, 57 R&D institutions involved, with related clinical trials reaching 71, and as many as 15539 patents.
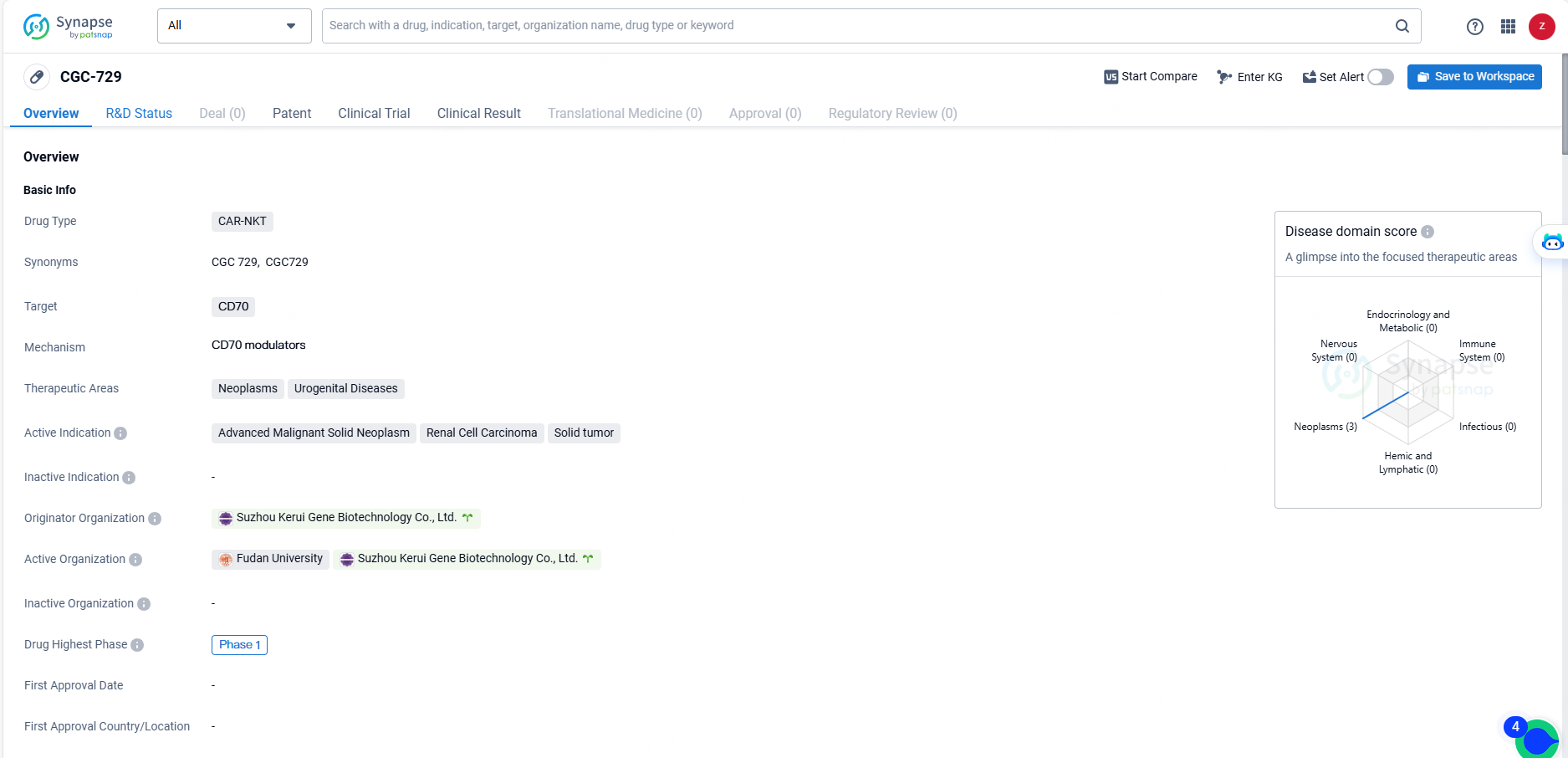
CGC-729 is a CAR-NKT type drug that targets CD70 and is being developed for the treatment of neoplasms and urogenital diseases, with a focus on advanced malignant solid neoplasm, renal cell carcinoma, and solid tumor.