Cytonics Initiates Phase 1 Trial of CYT-108 for Knee Osteoarthritis
Cytonics Corporation, a privately held company specializing in biotechnology and focused on developing biological treatments for musculoskeletal disorders, has announced the start of participant enrollment for its Phase 1 clinical trial of CYT-108. This trial will investigate a recombinant form of the alpha-2-macroglobulin blood serum protein, designed to inhibit proteases that contribute to cartilage wear and tear in osteoarthritis.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
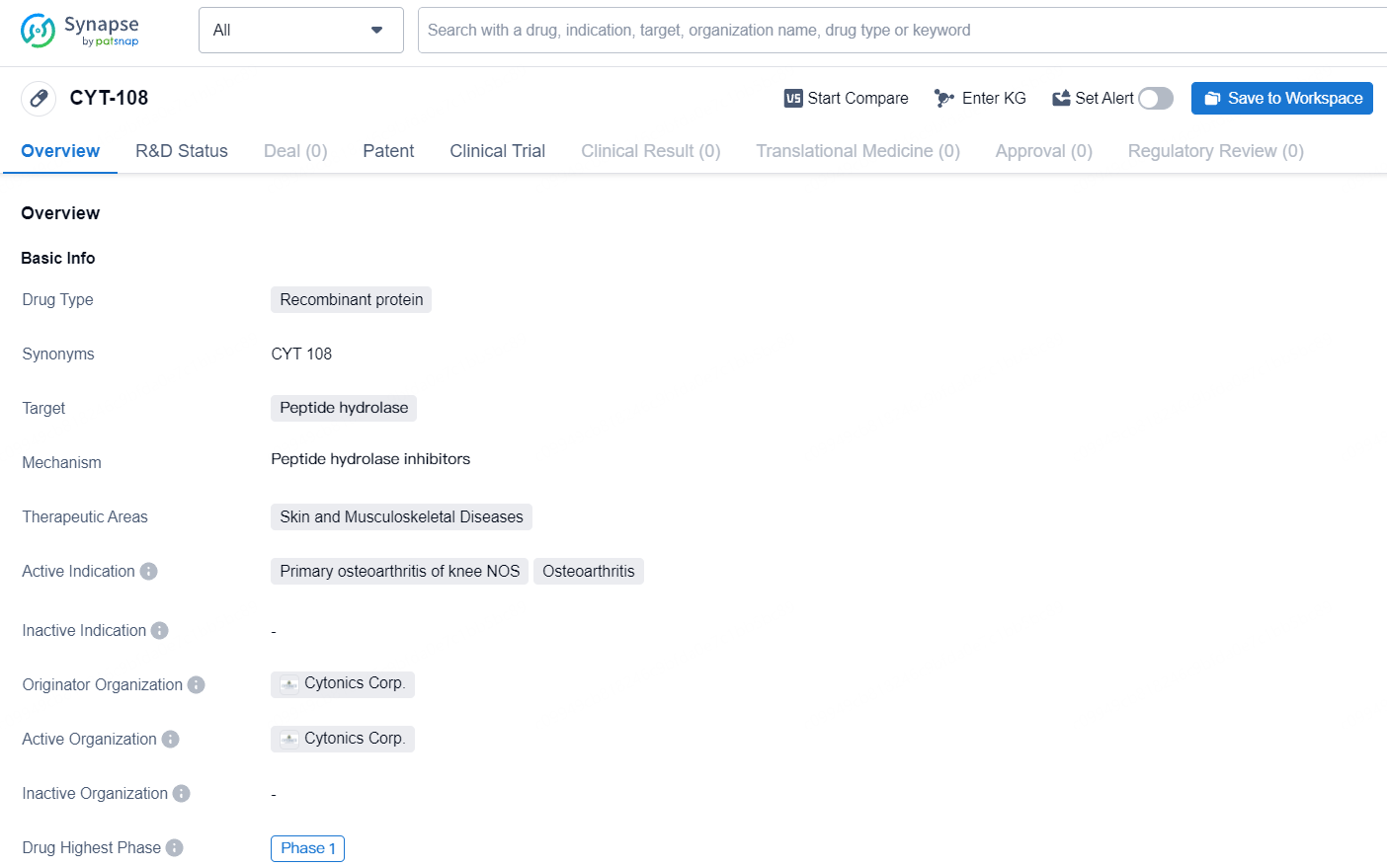
CYT-108 was designed to enhance its ability to inhibit proteases across the major classes that are primarily responsible for cartilage degradation in arthritic joints, while preserving its broad-spectrum anti-protease function against serine and threonine proteases.
"Our inaugural human clinical trial marks the peak of six years of preclinical research and development, during which we surmounted numerous technical obstacles in creating our recombinant A2M variant, CYT-108," stated Joey Bose, President & CEO of Cytonics.
"The preclinical results are promising, revealing CYT-108’s protective effects not only in cartilage but also in other joint structures involved in the development of osteoarthritis. Moreover, we believe the development risk of CYT-108 is mitigated by the clinical and commercial success of our first-generation therapy for osteoarthritis, the Autologous Protease Inhibitor Concentrate device, which has treated over 8,000 patients using autologous A2M selectively concentrated from their blood," added Joey Bose.
The Phase 1 trial is intended to evaluate CYT-108's tolerability and effectiveness in patients with primary knee osteoarthritis. The drug will be given intra-articularly at two different time points, 90 days apart. Patients will be regularly monitored for another 90 days to assess safety, pharmacokinetics, and effectiveness in alleviating joint pain, stiffness, and mobility issues.
CYT-108 is a recombinant version of the natural alpha-2-macroglobulin protein found in blood serum. The "bait" region of this protein, which acts as a substrate for proteases, was modified to boost its affinity for particular proteases implicated in the molecular onset of osteoarthritis.
The combination of highly specific and broad-spectrum protease inhibition activity makes CYT-108 stand out from other approaches that attempt to develop small molecule inhibitors targeting individual proteases, making CYT-108 a potential disease-modifying treatment for osteoarthritis.
The development of CYT-108 builds upon the success of Cytonics' initial therapy, the Autologous Protease Inhibitor Concentrate system, a 510(k) medical device that selectively concentrates autologous A2M for injection into joints with articular cartilage.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
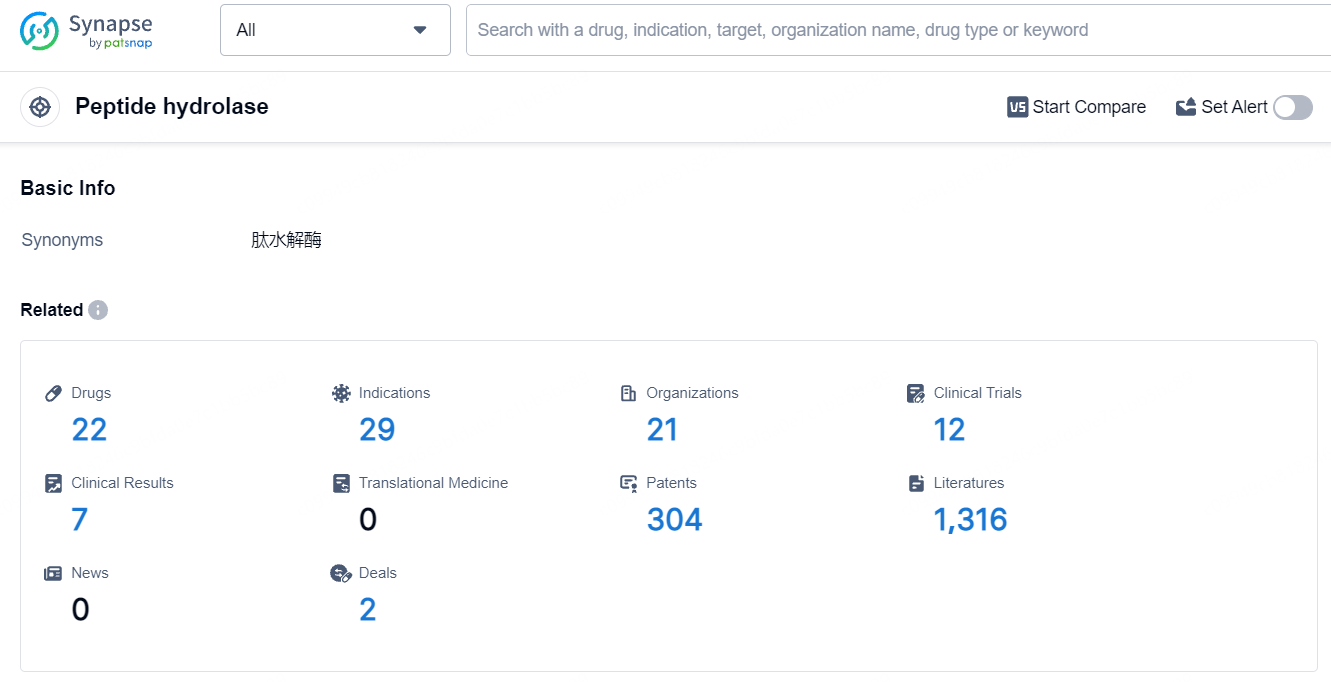
According to the data provided by the Synapse Database, As of July 18, 2024, there are 22 investigational drugs for the peptide hydrolase target, including 29 indications, 21 R&D institutions involved, with related clinical trials reaching 12, and as many as 304 patents.
CYT-108 is a recombinant protein drug that targets peptide hydrolase and is intended for the treatment of skin and musculoskeletal diseases, particularly in the context of primary osteoarthritis of the knee NOS and osteoarthritis. The drug is currently in Phase 1 of clinical development, indicating early-stage testing in humans.