Is Maribavir approved by the FDA?
Maribavir received FDA approval on November 23, 2021. This approval provides a new treatment option for post-transplant patients dealing with refractory CMV infections, particularly those that do not respond to standard antiviral therapies.
Usage and Dosage
Maribavir is administered orally in the form of 200 mg tablets, with the usual dosage being 400 mg taken twice a day. It can be taken with or without food. The treatment is designed to be continuous, even if symptoms improve, to prevent the virus from becoming resistant.
Side Effects
Common side effects of maribavir include:
- Vomiting
- Nausea
- Diarrhea
- Tiredness
- Changes in the sense of taste
Serious side effects can also occur and require immediate medical attention:
- Low blood cell counts, leading to fever, chills, tiredness, mouth and skin sores, easy bruising, unusual bleeding, pale skin, cold extremities, or feeling light-headed or short of breath
- Kidney problems, indicated by little or no urination, swelling in the feet or ankles, and feeling tired or short of breath
Patients should seek emergency help if they experience any signs of an allergic reaction such as hives, difficulty breathing, or swelling of the face, lips, tongue, or throat.
Precautions
Before taking maribavir, patients should inform their doctor if they have end-stage kidney disease, severe liver disease, or if they are pregnant or breastfeeding. Some medications can interact with maribavir, such as carbamazepine, phenytoin, and phenobarbital, potentially altering its effectiveness or increasing side effects. Patients should always follow their doctor's instructions regarding any restrictions on food, beverages, or activities while on maribavir.
Conclusion
Maribavir (Livtencity) is an FDA-approved antiviral medication specifically for treating refractory CMV infections in post-transplant patients. Its approval in November 2021 offers hope for patients who have limited options due to resistance to other antiviral treatments. As with any medication, it is crucial to use maribavir under the guidance of a healthcare professional, being mindful of potential side effects and drug interactions.
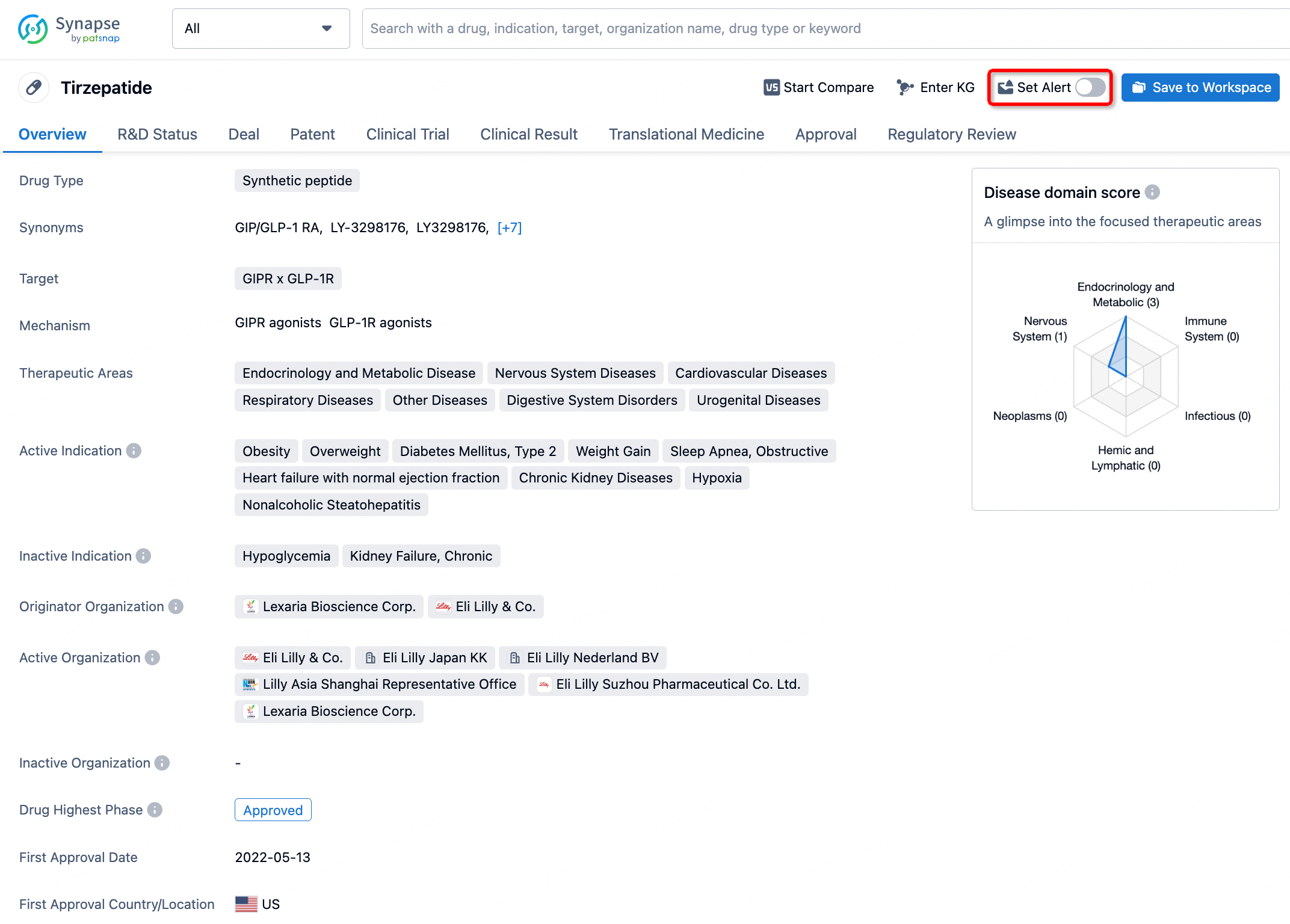
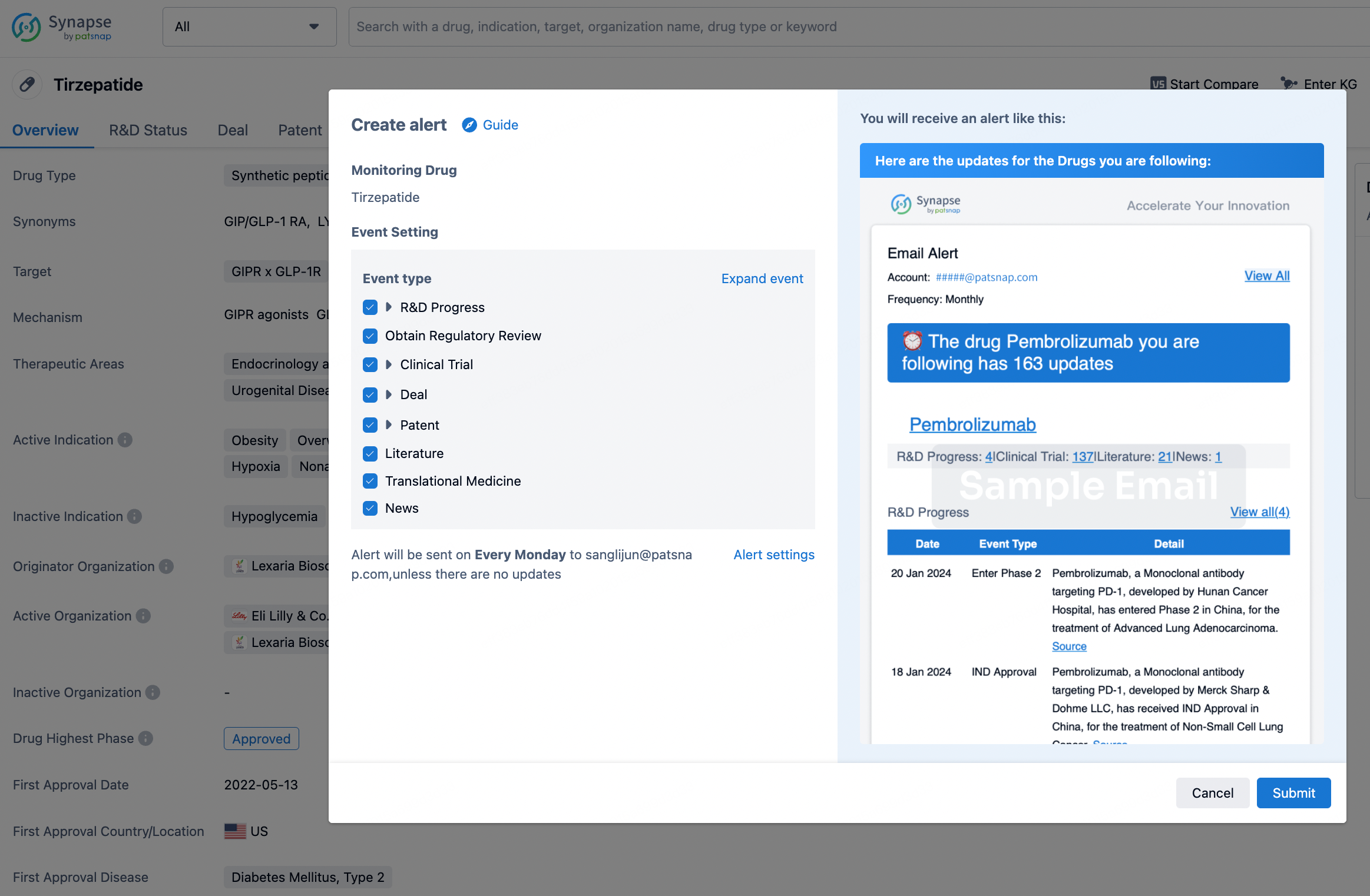
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!