DA-1726 Outperforms Survodutide in Pre-Clinical Weight Reduction and Health Metrics
NeuroBo Pharmaceuticals, Inc., a biotechnology company in the clinical stage specializing in the transformation of cardiometabolic disorders, revealed pre-clinical findings that suggest DA-1726, a new dual oxyntomodulin analog that acts as an agonist for both the glucagon-like peptide-1 receptor (GLP1R) and the glucagon receptor (GCGR), exhibited greater efficacy in weight reduction, maintenance of lean body mass, and lipid-lowering benefits compared to survodutide in pre-clinical models.
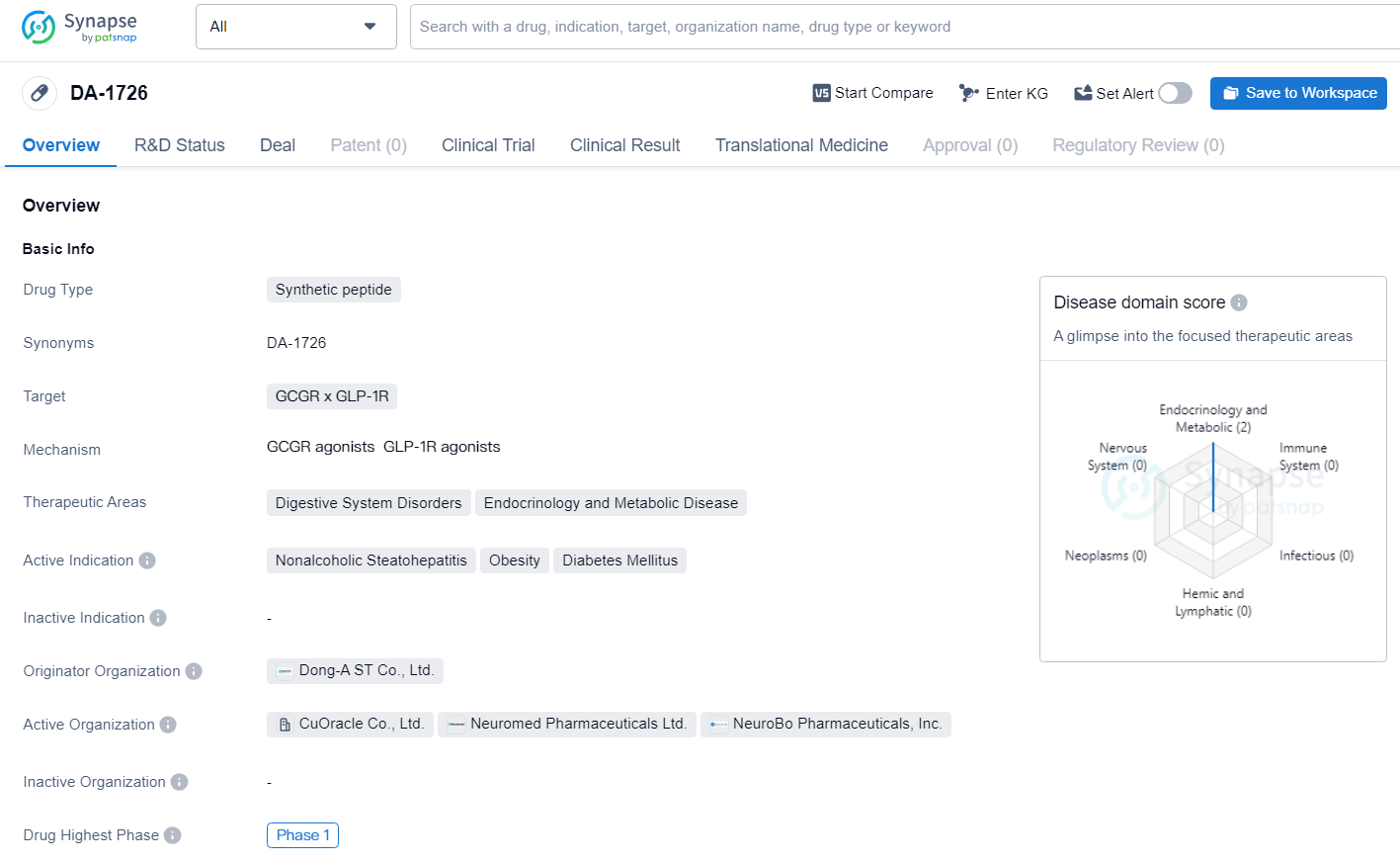
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
“The data presented at the ADA highlights the unique aspects of DA-1726 compared to other obesity drugs in the same category, likely due to its specific GLP-1 and glucagon receptor activity ratio,” said Hyung Heon Kim, President and CEO of NeuroBo. “We believe these unique features could potentially make DA-1726 a leading obesity treatment with enhanced efficacy and a better tolerability profile. The Phase 1 trial of DA-1726 is progressing smoothly; we anticipate dosing the first patient in the multiple ascending dose (MAD) Part 2 and releasing top-line data from the single ascending dose (SAD) Part 1 in the third quarter of this year, with MAD Part 2 top-line results expected in the first quarter of 2025,” Kim elaborated.
In obese mouse models, DA-1726 significantly reduced cholesterol levels and induced greater weight loss than survodutide, a drug with similar mechanisms, while also achieving better glucose reduction. Importantly, DA-1726 resulted in superior weight loss and maintained more relative lean body mass compared to survodutide.
Additionally, previous pre-clinical research demonstrated that DA-1726 could achieve similar weight reductions while allowing for higher food intake compared to tirzepatide. The new data presented at the ADA in a hypercholesterolemia rat model further confirmed DA-1726's superior effectiveness over tirzepatide in reducing cholesterol levels due to its glucagon properties, alongside its GLP-1 effect, and in preventing weight gain.
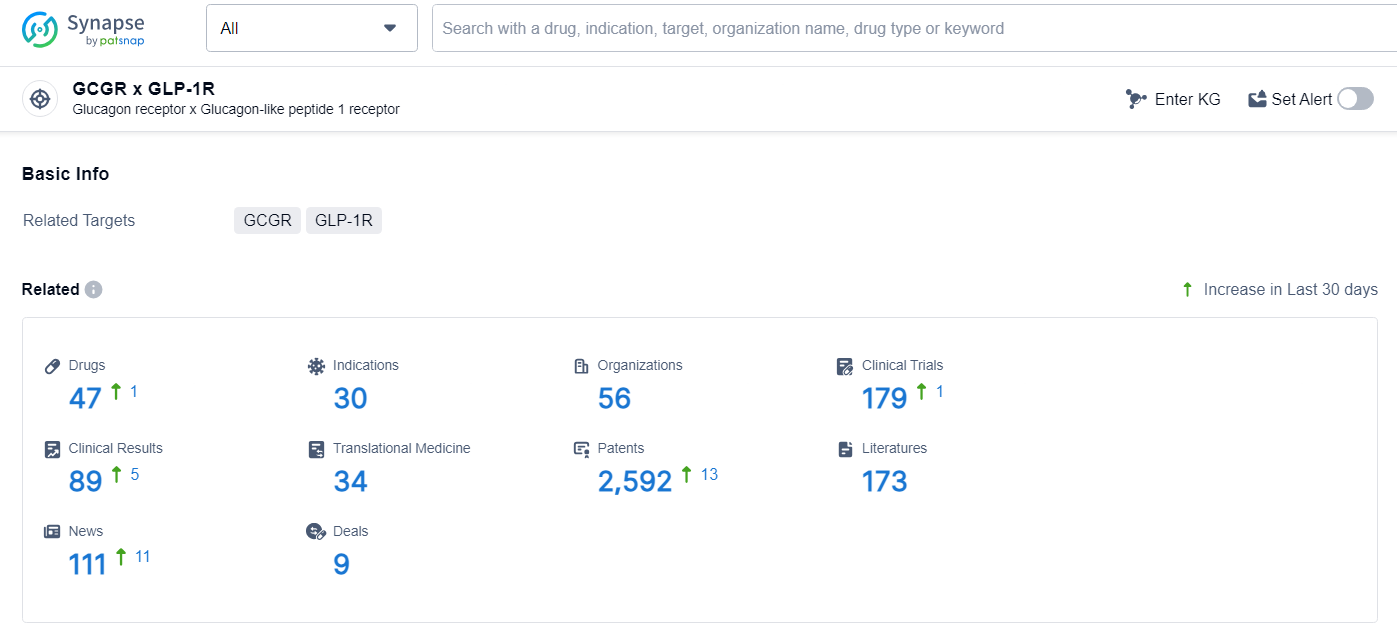
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of June 27, 2024, there are 47 investigational drugs for the GCGR and GLP-1R target, including 30 indications, 56 R&D institutions involved, with related clinical trials reaching 179, and as many as 2588 patents.
DA-1726 is a synthetic peptide drug with a focus on targeting GCGR x GLP-1R for the treatment of digestive system disorders, endocrinology, and metabolic diseases. While still in the early stages of development, the drug shows promise in addressing critical medical needs in nonalcoholic steatohepatitis, obesity, and diabetes mellitus. As it progresses through clinical trials, DA-1726 has the potential to make a significant impact in the field of biomedicine.